
The relationship between antibiotics and drug-resistant bacteria is an ongoing battle. In recent years, antibiotic resistance has become a global public health issue. What appears to be an endless cycle of combat may be disrupted by a new therapeutic approach - bacteriophage therapy.
In fact, bacteriophage therapy is not entirely new; it predates antibiotics and has been in use for some time. However, its application as an antibacterial agent faced certain limitations, including numerous unknown factors, operational complexities, and a narrow antibacterial spectrum. The emergence of synthetic biology has opened up new possibilities for bacteriophage therapy.

(Source: Nucleic Acids Research)
Recently, Ma Yingfei's team at the Shenzhen Institutes of Advanced Technology, CAS, published a paper titled "Genome-scale top-down strategy to generate viable genome-reduced phages" in the international academic journal "Nucleic Acids Research" (IF=19). It presents a high-throughput method for preparing chassis phages while identifying non-essential genes of the phages. It results in genome-reduced phages with stronger infectivity than wild-type phages, holding tremendous potential in bacteriophage therapy and synthetic biology. Ma Yingfei, is the correspondent author, and his Ph.D. student Yuan Shengjian is the first author of the paper.
The research received support from the Ministry of Science and Technology Key R&D Program, the Chinese Academy of Sciences Pilot B Program, CAS Key Laboratory of Quantitative Engineering Biology, the Guangdong Synthetic Genomics Key Laboratory, and the Shenzhen Institute of Synthetic Biology.

▲ Picture 丨 Ma Yingfei (Source: Interviewee)
Ma Yingfei earned his Ph.D. from the Institute of Microbiology, Chinese Academy of Sciences, in 2009. He has previously worked on major projects related to the Human Microbiome Project (HMP) at the University of California, San Diego, and the New York University School of Medicine. He joined the Shenzhen Institutes of Advanced Technology in 2015, where his team focuses on basic and applied research involving bacteriophages.
High-throughput Preparation of Chassis Phages
Bacteriophages are organisms with the highest diversity and abundance on Earth, serving as essential models in synthetic biology research. By deleting redundant genes from the genome, chassis phages can expand their DNA packaging capacity, carry more functional genes, enhance their antibacterial activity and potential in various bioengineering applications. This process also deepens our understanding of bacteriophages.
Currently, high-throughput deletion of redundant genes in bacteriophages remains challenging for researchers. First, how can we realize high-throughput identification of the non-essential genes in bacteriophages? Second, how can we screen mutant phages with growth advantages? Third, how can we simultaneously screen mutant phages that can maintain robust growth while deleting genes from different regions of the genome, given the labor-intensive and time-consuming nature of this process?
The CRISPR system is a bacterial defense mechanism against phages. When bacteria infected by phages and containing artificially designed CRISPR systems are used, there is a chance of deleting a specific phage gene (mutant strain 1). If mutant strain 1 is then used to infect host bacteria containing another artificially designed CRISPR system, there is a chance of deleting another phage gene (mutant strain 2). If this process continues, a chassis phage can be obtained.
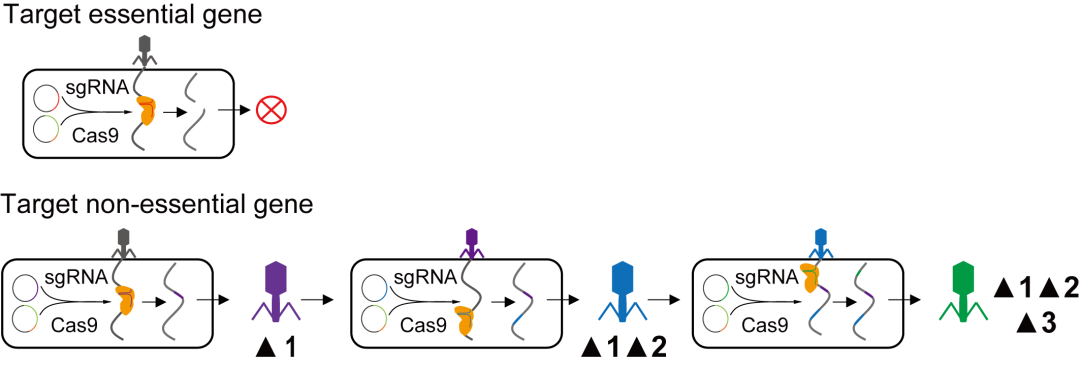
▲ Picture 丨 Schematic Diagram of Continuous Deletion of Phage Genes (Source: Interviewee)
Based on this, Ma Yingfei's team wondered if they could mix host bacteria with different artificially designed CRISPR systems, allowing high-throughput deletion of phage genes and the selection of mutant strains with strong infectivity.
The team developed the CRISPR/Cas9-based iterative phage genome reduction, CiPGr. For all target genes, different CRISPR systems were designed, which were synthesized on a DNA chip to create a CRISPR bacteria library for deleting different phage genes. In the CiPGr process, a swarm of phages are used to infect bacteria library, leading to different phages deleting different genes, and forming a mutant phage library. If the deleted genes are non-essential for phage amplification, the descendants of mutant phages can continue to infect new bacteria. The mutant phage library is continually transferred to freshly cultured bacterial libraries, causing gene deletions to accumulate in the phage genome. Genome-reduced phages with growth advantages will eventually dominate the population and can be isolated and purified.
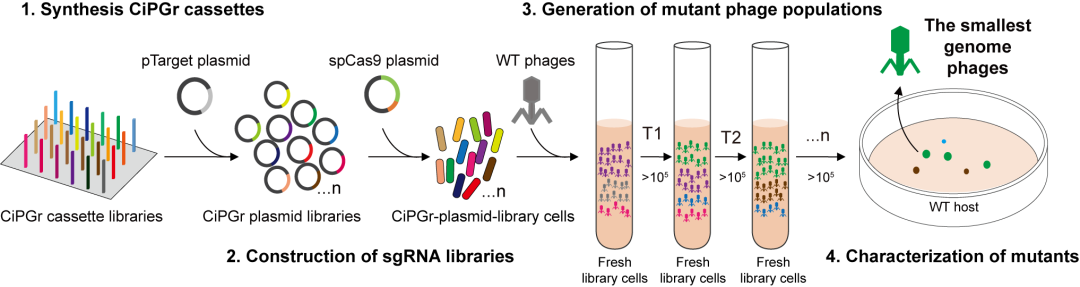
▲ Picture 丨 Experimental Process Design (Source: Interviewee)
Through CiPGr, the team successfully reduced the genomes of four different tailed phages (T7, T4, seszw, and selz), obtaining their chassis phages, non-essential gene sets, and genome-reduced phages with stronger infectivity than wild-type phages.
Based on bioinformatic analysis, next-generation genomic sequencing of the mutant phage library enabled the straightforward and efficient identification of deletable gene sets, constituting approximately 50% of the total gene count in the four phages.
The team found that some mutant strains had stronger bacteriostatic or bactericidal ability than wild-type ones, and isolated and identified these mutant strains. After that, the researchers mixed the obtained mutant phage library with wild-type host bacteria for culture, which confirmed that mutant strains with stronger lysis ability than wild-type phages could be obtained by genome reduction.
The team has also observed that the number of gene deletions accumulates in a monoclonal mutant phage genome as the transfer cycle increases. This finding indicates that in a monoclonal mutant phage genome, the earlier the gene is deleted, the less important it is for phage amplification. That is, the sequence of gene deletion is related to its order of importance in phage amplification.
In summary, this method has several key implications. First, it can be applied to other wild-type phages as long as their genome sequences are obtained during the CiPGr process, with no need for prior knowledge. Second, these genome-reduced phages can integrate more genetic elements to enhance their functionality, holding significant potential in phage therapy. Third, identifying essential, non-essential, and quasi-essential genes in phage genomes is of great significance for redesigning phage genomes and advancing phage biology research.
No adverse effects have been found in clinical applications.
Many compounds and antibiotics known to kill bacteria have varying degrees of toxicity to the human body. For instance, the antibiotic polymyxin, often regarded as the "last line of defense,” is highly nephrotoxic.
Since the discovery of phages, there have been successful cases of using them to treat bacterial infections.
In 2019, a case of using bacteriophages to treat superbug infections was published in Nature Medicine. In this case, a 15-year-old boy, who had developed an infection with Mycobacterium abscessus, a nearly untreatable superbug, after undergoing a bilateral lung transplant, was successfully treated with bacteriophage therapy. Through genome engineering and forward genetics, doctors identified three bacteriophages, which successfully cleared the superbug from the patient's body.
Currently, bacteriophage therapy in China is still in the clinical trial stage, but there have been many successful cases.
After Professor Yu He's bacteriophage therapy case in 1958, the second successful bacteriophage therapy case in China took place in 2018 at the Shanghai Public Health Clinical Center (Southern Branch of Zhongshan Hospital). In this case, the patient, who was cured through bacteriophage therapy, ended years of suffering from a drug-resistant Klebsiella pneumoniae urinary tract infection, making it the first bacteriophage therapy case in China approved by ethics review.
Ma Yingfei also revealed to SciPhi-SynBio that "we are currently validating the effectiveness of bacteriophage therapy through large-scale animal experiments, including highly representative model organisms such as mice, rats, and monkeys. From the preliminary results, it appears to be a very safe antimicrobial agent, causing no significant immune reactions in animals, both in terms of host safety and the host's metabolism.”
In 2020, Ma Yingfei's team successfully conducted the first clinical trial of bacteriophage therapy for drug-resistant Acinetobacter baumannii lung infections in Shenzhen People's Hospital. So far, there have been multiple successful human clinical cases where bacteriophage therapy was used and drug-resistant bacterial infections in the lungs were successfully cleared.
As a new alternative to antibiotics, there will be a significant demand for bacteriophages in the market.
"We conducted a survey in a Grade 3A hospital, and the results showed that the hospital had thousands of patients with clinically diagnosed drug-resistant bacterial infections each year. This suggests that there is a huge demand for new antibiotic alternatives nationwide. Currently, bacteriophage therapy is often considered only when there are no other cures for drug-resistant bacteria. If we can intervene early, it may improve the clinical situation of drug-resistant bacterial infections," said Ma Yingfei.
Currently, bacteriophage therapy, in addition to its application issues clinically and in breeding, also requires criteria and specifications for clinical use.
“At present, there are no clear clinical or usage specifications for bacteriophage drugs or feed additives. I hope that after some innovative research on bacteriophages in the future, we can further push for breakthroughs in regulatory agencies to enable the large-scale application of bacteriophage drugs. I personally have high expectations for the market value of bacteriophage therapy,” Ma Yingfei stated.
Currently, both domestic and international synthetic biology research on bacteriophages is relatively synchronized in some cutting-edge areas. Also, the use of bacteriophage therapy in clinical applications and breeding faces similar challenges. "Internationally, some companies are already developing bacteriophage therapy applications, such as APT in the United States, which is now in Phase I and Phase II clinical trials. We are in a close pursuit state and hope to catch up with or even surpass them in the future," Ma Yingfei concluded.
Finally, Ma Yingfei expressed great optimism about the future of synthetic biology. "We often say that the 21st century is the century of biology, but I think it is more accurate to say that it is the century of synthetic biology. Synthetic biology research on bacteriophages allows us to redesign, synthesize, and give them new functions, making bacteriophages more powerful. In the future, synthetic biology will play a significant role in driving the application of bacteriophage therapy.”
Reference hyperlink: 1. https://academic.oup.com/nar/advance-article/doi/10. 1093/nar/gkac1168/6895194