
On June 8, Beijing time, Ma Yingfei's research group of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, published a paper in Nucleic Acids Research, an international journal, under the title of “Global transmission of broad-host-range plasmids derived from the human gut microbiome”. In this work, a large number of human intestinal plasmids were deeply analyzed and revealed, and a series of unknown Broad host range (BHR) plasmids were identified for the first time. It was also found that BHR plasmids could carry adaptive genes and spread widely in various environments around the world, which provides a new insight into the intestinal ecosystem and the transmission of drug resistance genes.

Screenshot of the published paper
Link of the paper: https://doi.org/10.1093/nar/gkad498
Plasmids are mobile genetic elements outside the chromosomes of microorganisms. The gut microbiome contains a large number of gut plasmid groups and is an important participant in horizontal gene transfer events in the intestinal ecosystem. The BHR plasmids carrying adaptive genes can transfer significantly across phylogenetic groups, which promotes different hosts to adapt to different environments. The study of the transmission mechanism of BHR plasmids and the elements they carry is of great significance for understanding the evolution of flora, the global transmission of antibiotic resistance, and the engineering of plasmid elements.
In the past, scientists isolated a small number of BHR plasmids from clinical and other environments and characterized them, but due to the lack of large-scale and systematic research, people still understand them very little. The development of bioinformatics has allowed researchers to analyze and assemble plasmid sequences on a large scale from metagenomes, but existing tools are still insufficient to accurately analyze the host range of gut plasmids and identify BHR plasmids. In this study, based on the draft genome of human intestinal bacterial isolates obtained by culturomics, plasmid-like sequences (PLSs) carried by the isolates were identified and characterized, and their accurate host range, environmental stability and universality, carried genes, as well as transmission and evolutionary traces were analyzed.
Novelty and diversity of human gut plasmids
In this study, the research team developed an analytical method for identifying PLSs (Figure 1A). With the draft genome of human intestinal bacterial isolates as a sample, the team screened a large number of PLSs and found that they were widely distributed in bacterial hosts of different genera (Figure 1B). These PLSs could then be grouped into plasmid-like clusters (PLCs). The plasmid skeleton genes responsible for replication, mobilization, conjugation, or stabilization were further examined, and their size and integrity were identified (Figure 1C). The team further compared high-integrity (>60%) PLCs with plasmid sequences in public databases, constructed a complete sequence similarity network, and divided the sequences into 103 network typing groups (Figure 1D and 1E). The results suggested that the network layout was consistent with the replicon types. The analyzed PLCs had several replicon types, many of which were unknown. They formed several independent network typing groups, demonstrating the novelty and diversity of PLCs identified in the human gut microbiome.
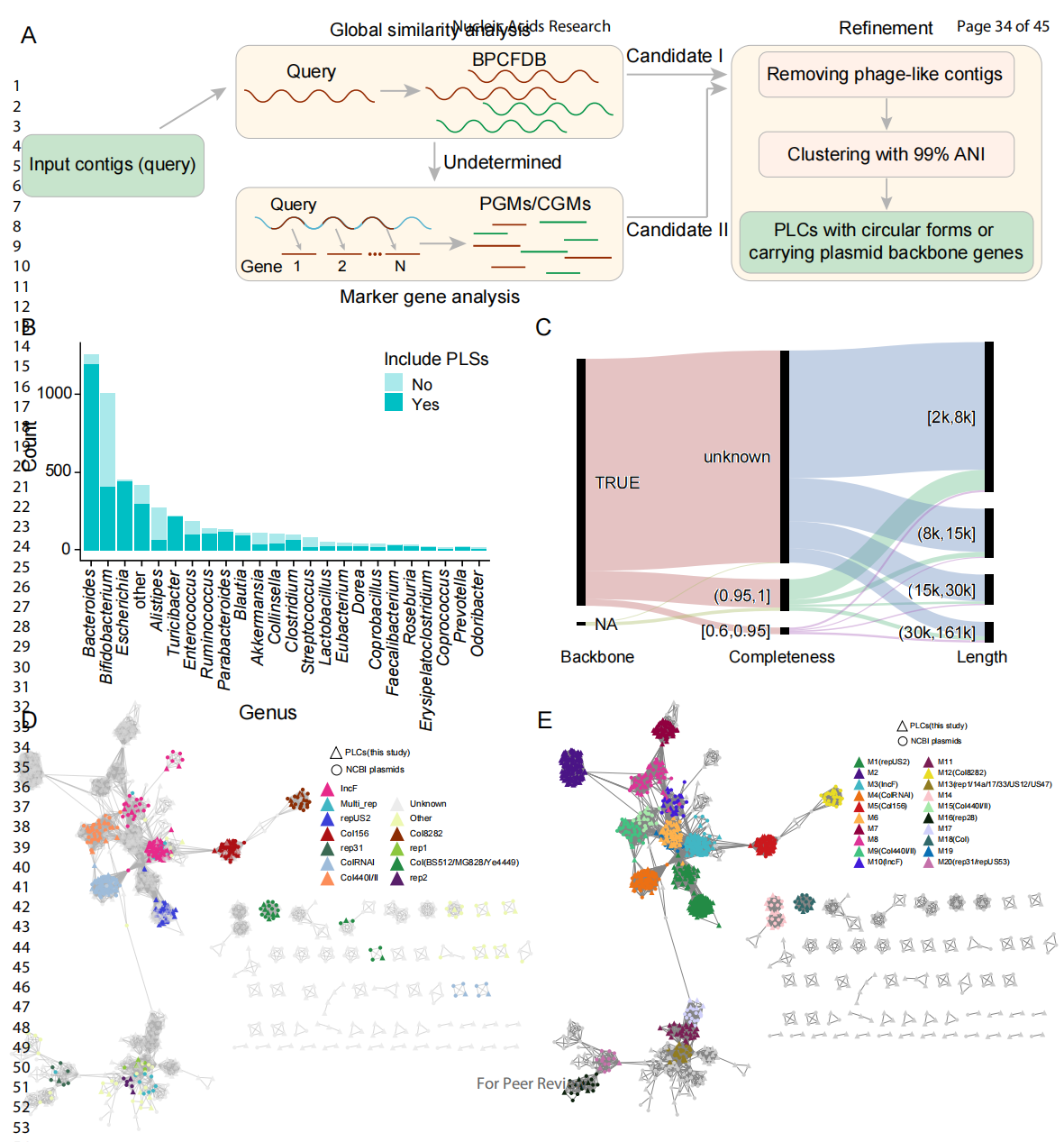
Figure 1 Identification and characteristic analysis of PLSs in human intestinal bacterial isolates
Human gut plasmids are characterized by a broad host range
With gut host samples from different countries as reference, the team identified 402 PLCs that could transmit across genera in the aforementioned PLSs set, some of which were detected in samples from different countries. On the premise of diversity, the team analyzed 175 representative PLCs with broad host range and found that Bacteroides, Escherichia, and Bifidobacterium were the most common hosts of these PLCs with broad host range, and different Groups showed different host distribution differences (Figures 2A, 2B, and 2C). The PLCs with the broadest host range (Clstr_417) could transmit across 5 phyla and 24 genera. The team also found that 61.2% of the tested PLCs with broad host range could remain stable for at least 150 days, or were the basis for their wide transmission (Figure 2D).
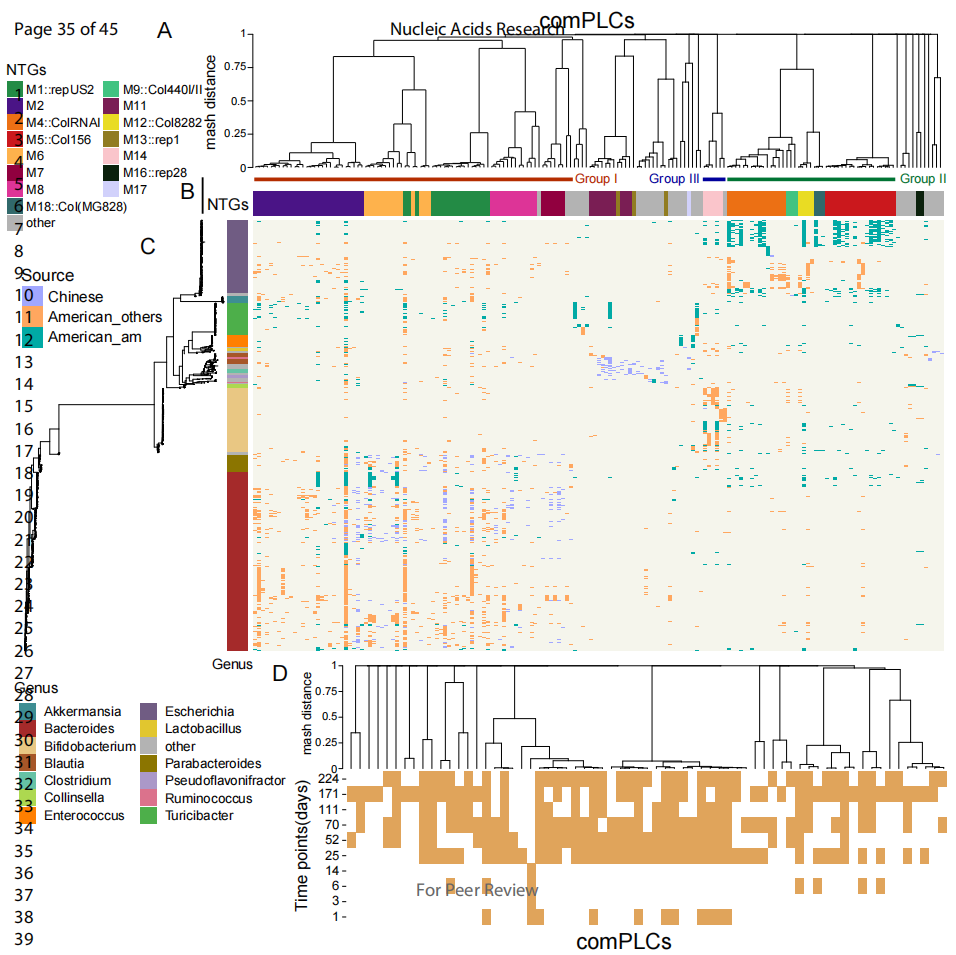
Figure 2 Distribution and persistence of human hut plasmids across phylogenetic and geographical regions
Human gut plasmid encoding adaptive genes
Since plasmids often carry adaptive genes, the team focused on analyzing antibiotic resistance genes, virulence genes, and carbohydrate active enzyme genes encoded by human gut plasmids (Figure 3). The results showed that 6.3% (339) of the analyzed PLCs carried drug resistance genes, with individual PLCs even carrying up to 7 drug resistance genes. Different types of antibiotic genes exhibited different host distribution differences, suggesting that the gut microbiome could serve as a resistance gene pool. Similar host-specific distributions were also observed on carbohydrate active enzyme genes with Bacteroides being the most common host. Escherichia coli was the host carrying the most virulence gene PLCs (95.8%). Interestingly, possibly due to their larger genome, conjugative BHR plasmids carried these three types of adaptive genes more frequently than mobile BHR plasmids.
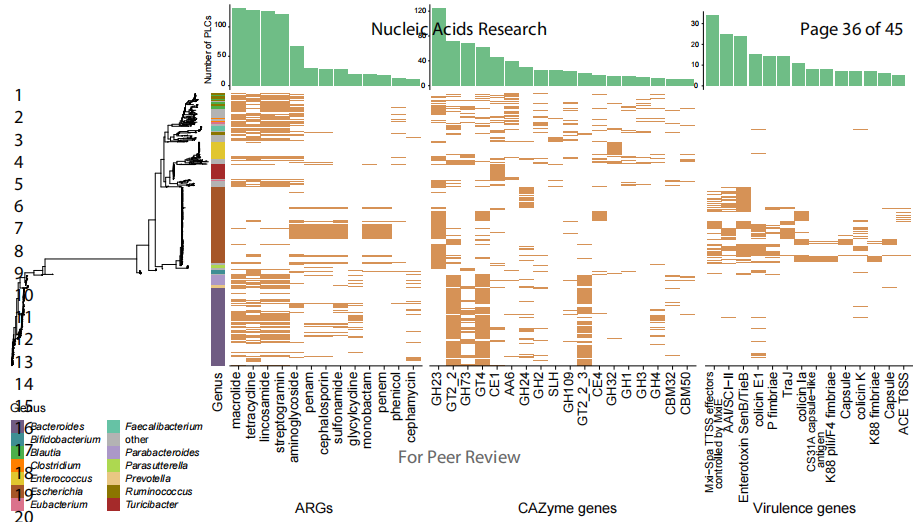
Figure 3 Distribution of PLCs accessory genes in PLSs
Human gut plasmids transmit widely around the world
With metagenomic samples from human gut, swine gut, and sewage plants in different countries as reference, the team found that 22.91% of PLCs could be present in different environmental niches (Figure 4A). PLCs were also widely present across populations (Figure 4B), and different living habits could cause differences in the distribution of PLCs species (Figure 4C). In particular, 13 BHR plasmids were highly prevalent in different populations (prevalence >10%), among which the representative BHR plasmid Clstr_599 was present in all populations with a prevalence greater than 16.5% and appeared in all environmental niches (Figure 4D). The BHR plasmids also carried antibiotic resistance genes that were widely present in different population and environmental samples, which may greatly promote the transmission of ARGs and other suitable genes in various environments (Figure 4E). By constructing a haplotype network, the team also analyzed the evolution and transmission traces of the representative plasmid Clstr_599, and found that it transmitted extremely rapidly between the bacteriophyta and the environment with all transmission times occurring 0 and 10 years ago and colonizing the population many times. The team also emphasized that it was the universality of the Clstr_599 replication mechanism that mediated the significant cross-host transfer of Clstr_599.
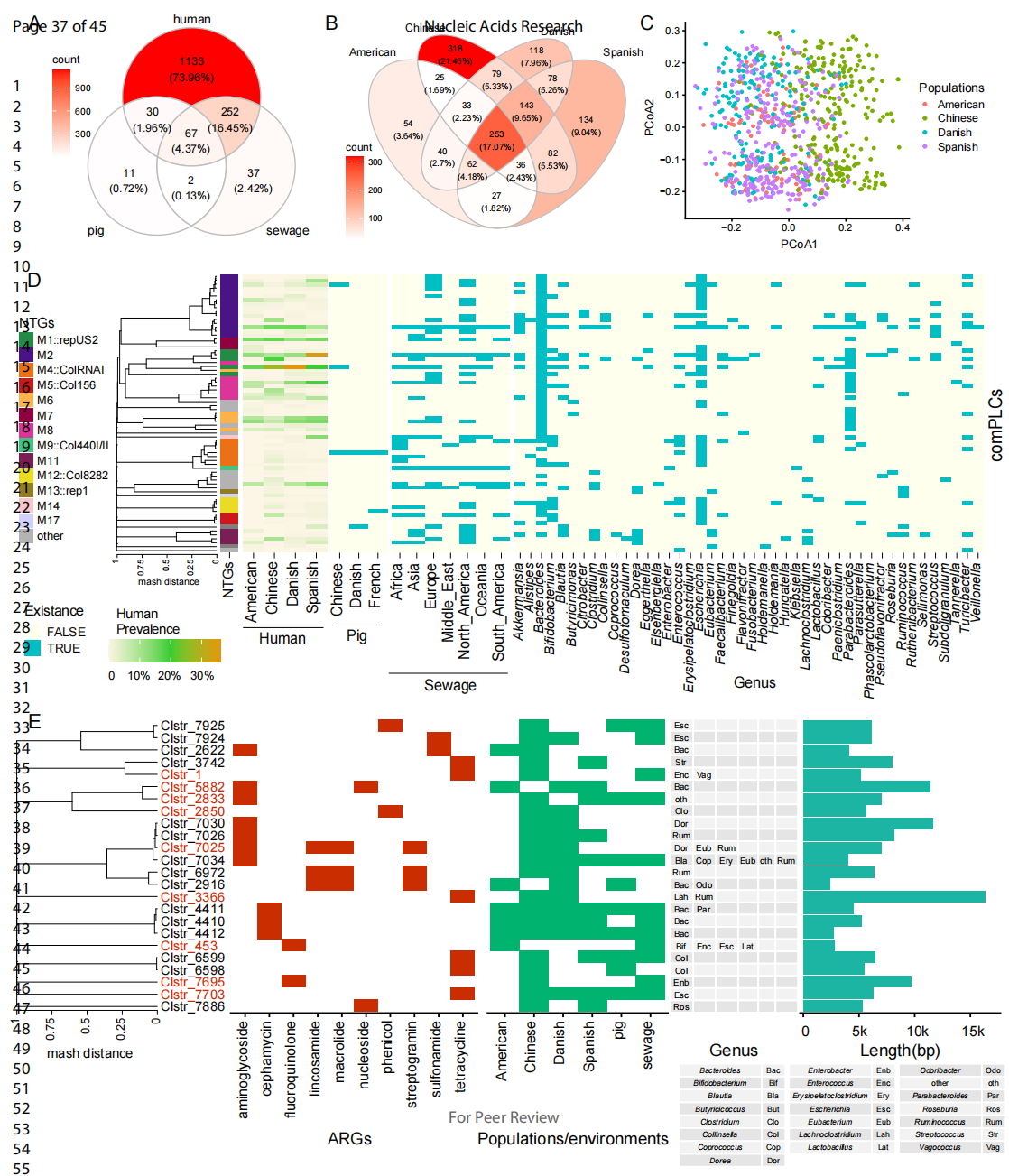
Figure 4 Prevalence of PLCs in different ecological niches and populations
In summary, this study systematically explored human gut plasmids and identified a series of plasmids with broad host range that transmitted widely around the world, which provided new insights into the diversity and novelty of human gut plasmids and highlighted the potential impact of plasmids on human health around the world. In addition, since most of the human intestinal bacteria are non-model bacteria, there is currently a lack of suitable gene editing tools for editing intestinal bacteria. The plasmids with broad host range discovered in this study will have great application potential in the field of genome editing of intestinal bacteria and phage.
Researcher Ma Yingfei of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, is the corresponding author of the paper, and Yang Lili is the first author of the paper. This research was supported by programs such as the Priority Program B of the Chinese Academy of Sciences (XDB29050501) and the Shenzhen Institute of Synthetic Biology. This research was also support and assisted by Zhou Haokui, Hu Zheng, and Dai Lei of Shenzhen Institute of Advanced Technology, as well as Deng Ziqing of BGI.