
On May 8, the latest achievement of Yan Fei, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, was published in Signal Transduction and Targeted Therapy, an international well-known journal, under the title of “Cell-cycle dependent nuclear gene delivery enhances the effects of E-cadherin against tumor invasion and metastasis”. In this work, the research team developed a new method for in vivo spatiotemporally controllable intranuclear gene delivery. In this method, Gas vesicles (GVs) were used as cavitation nuclei to carry plasmid DNA. After endocytosis, the gene-loaded Gas vesicles were induced by ultrasound to produce cavitation effect in the cell, which can directly deliver plasmid DNA to the nucleus to realize gene transcription and expression. Given that ultrasound has the characteristic of targeted focusing and Gas vesicles can stay in cells for a long time due to their natural biocompatibility, spatiotemporally controllable intranuclear gene delivery could be realized. By using the E-cadherin gene, which inhibited tumor metastasis, as an example, the research team demonstrated that gene delivery and expression could be controlled in different time dimensions, and tumor invasion and metastasis could be inhibited by using the developed intracellular cavitation intranuclear gene delivery. The team compared the delivery of E-cadherin gene in different cell cycles by this method and found that controlling the intranuclear delivery and expression of exogenous gene E-cadherin in the G2/M phase of tumor cells was more effective in inhibiting tumor invasion and metastasis than in the G1 and S phases. Further molecular mechanism studies showed that the Fam50a/Runx2-MMP13 signal axis played an important role in the enhancement of this effect.
This work can not only effectively improve the transfection efficiency of exogenous genes, but also is expected to be developed into an in vivo spatiotemporally controllable tool to control the expression of exogenous genes, which has important application prospects.
Yan Fei, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, and Jiang Tian'an, director of The First Affiliated Hospital, Zhejiang University School of Medicine, are the co-corresponding authors of the paper. Xie Liting, a visiting student of Shenzhen Institute of Advanced Technology from Zhejiang University, is the first author of the paper.

Screenshot of the published paper
Link of the paper: https://www.nature.com/articles/s41392-023-01398-4
In recent years, gene therapy has been regarded as a new therapeutic method with great application potential in addition to surgery, radiotherapy, and chemotherapy. Ultrasound targeted microbubble blasting technology is a novel gene and drug delivery technology. Its principle is to use ultrasound contrast agent microbubbles as gene vectors, induce the gene-loaded microbubbles to periodically contract, expand and even collapse and blast by ultrasound local irradiation, and then produce a series of cavitation effects such as micro-flow, micro-jet, and shock wave. These effects superimpose on adjacent cell membranes, making them have instantly repairable tiny pores (sonoporation effect), and pasmid DNA in the solution or released by microbubble blasting takes the opportunity to enter the cells, thus realizing site-directed gene delivery. This method has the advantages of targeting, site-directing, and visual gene delivery, and is one of the promising methods for gene targeted delivery. However, conventional ultrasound gene delivery strategies based on microbubble ultrasound contrast agents can only perform cavitation perforation on the cell membrane to deliver exogenous genes from outside the cell to inside the cell, but cannot directly deliver plasmid DNA into the nucleus, resulting in generally low efficiency of ultrasound targeted gene transfection. Aiming at this issue, researcher Yan Fei's team proposed a new method of intracellular cavitation intranuclear gene delivery based on Gas vesicles. They took advantage of the small particle size of Gas vesicles to make them carry plasmid DNA through PEI surface modification, then incubate them with the cell into the cytoplasm. After that, they make the vesicles produce cavitation effect in the cell by ultrasound irradiation, and the instantaneously perforated nuclear membrane directly delivered genes into the nucleus, which effectively improved the transfection efficiency of genes (up to 47% by flow cytometry). In addition, by utilizing the natural compatibility of Gas vesicles and their stable presence in cells, the intracellular delivery and expression of genes could be controlled by ultrasound at different time points, which successfully realized in vivo spatiotemporally controllable gene expression of exogenous gene.

Figure 2. Ultrasound visual intracellular cavitation EGFP gene delivery controlled the expression of exogenous genes in the time dimension
The research team used this system to deliver the E-cadherin gene that can inhibit tumor metastasis and found that after endocytosing E-cadherin-loaded vesicles in C6 glioma cells, the transplantation of these vesicles into the mouse brain and waiting for 0 h (t0), 12 h (t12), or 24 h (t24) for ultrasound irradiation to induce intracellular cavitation of the Gas vesicles for intranuclear gene delivery could effectively inhibit tumor cell migration and extended the survival period of tumor-bearing mice. To explore the application of ultrasound intracellular cavitation intranuclear gene delivery in the cell cycle, the research team established two different cell cycle operation modes, namely, synchronizing tumor cells to stay in different cell cycles for ultrasound gene transfection and synchronizing cells, removing cell cycle the blocker, and receiving ultrasound gene transfection after entering different cell cycles (the tumor cells first endocytosed gene vesicles). The experimental results demonstrated that the developed intranuclear gene delivery method had similar gene transfection efficiency in the G1, S, and G2/M phases of the two cell cycle operating modes, but differed in the way of inhibiting tumor cell invasion and metastasis. This was manifested by the better anti-tumor invasion and metastasis effect of ultrasound intranuclear gene delivery in the G2/M phases than in the G1 and S phases.
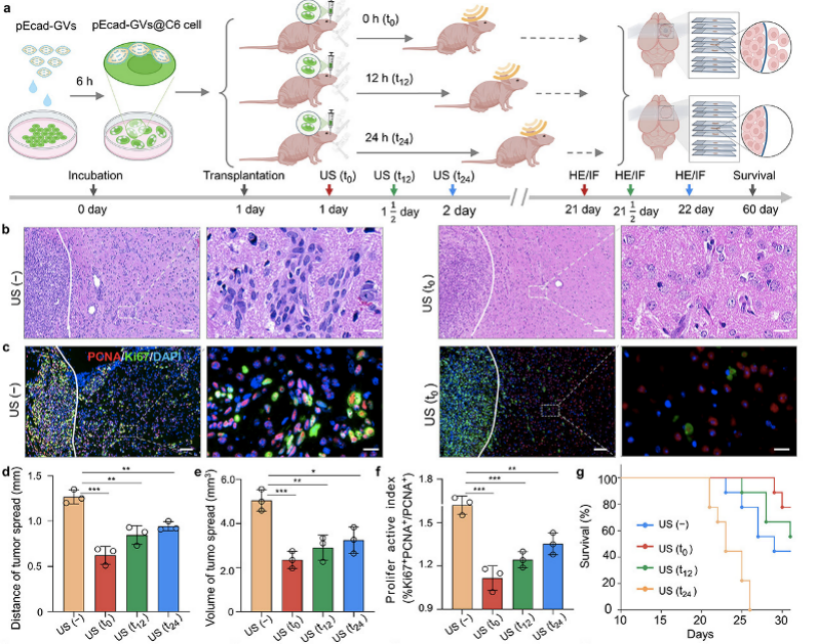
Figure 3. Ultrasound intracellular cavitation effectively inhibited the migration of glioma cells by delivering E-cadherin at different times

Figure 4. Transfection efficiency of ultrasound intracellular cavitation in delivering EGFP at different cell cycles and tumor migration inhibition effect of delivering E-cadherin
To further explore the mechanism by which ultrasound intracellular cavitation delivery of E-cadherin in the G2/M phase could better inhibit tumor metastasis, through molecular experiments such as transcriptome sequencing, qPCR, WB, and CO-IP, the research team found that overexpression of E-cadherin in the G2/M phase of tumor cells could lead to down-regulation of the Fam50a gene, then reduced the interaction and transcriptional activation function of Fam50a/Runx2, and ultimately led to down-regulation of MMP13, which revealed the mechanism by which ultrasound intracellular cavitation intranuclear delivery of E-cadherin gene better inhibited tumor metastasis in the G2/M phases of the cell cycle.
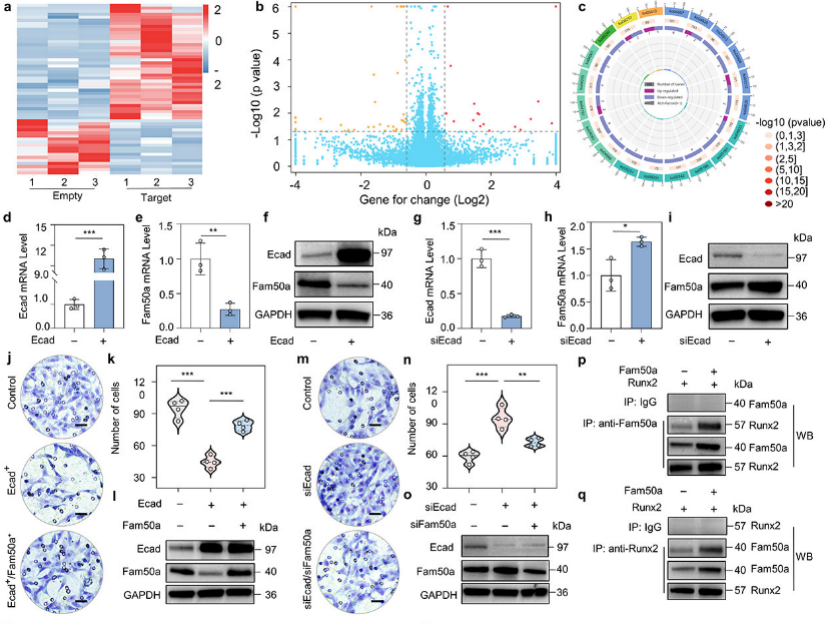
Figure 5. Overexpression of E-cadherin in the G2/M phases more effectively inhibited Fam50a/Runx2-MMP13 signaling
This work was supported by the Key Research and Development Program of the Ministry of Science and Technology of China, the National Natural Science Foundation of China, the Priority Program B of the Chinese Academy of Sciences, and programs of Shenzhen Science and Technology Innovation Committee, Shenzhen Institute of Synthetic Biology, etc.
Liting Xie, Jieqiong Wang, Liming Song, Tianan Jiang and Fei Yan. Cell-cycle dependent nuclear gene delivery enhances the effects of E-cadherin against tumor invasion and metastasis.Signal Transduction and Targeted Therapy, 2023; 8(1):182. doi: 10.1038/s41392-023-01398-4.