
On April 24, Tang Hongting, an associate researcher, and Luo Xiaozhou, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, jointly published the research achievement in Metabolic Engineering under the title of “Systematic genetic modifications of cell wall biosynthesis enhanced the secretion and surface-display of polysaccharide degrading enzymes in Saccharomyces cerevisiae”. In this work, based on synthetic biology strategies, researchers systematically explored the impact of key genes in the biosynthetic pathway of cell walls on the secretion and surface-display of BGL1 enzyme activity in Saccharomyces cerevisiae, screened new gene modification targets that could enhance recombinase activity, and combined and modified these new gene targets to further improve the activity of recombinase. Moreover, through proteomics and reverse engineering analysis, the study found that the up-regulated expression of key genes involved in protein translation and secretion pathways played an important role in improving the secretion and surface-display activity of recombinase. This study provides a new idea for constructing yeast cell factories to efficiently secrete and display recombinant proteins. The first authors of the paper are research assistant Chen Nanzhu, master degree candidate Yang Shuo, and research assistant You Dawei. Associate researcher Tang Hongting and researcher Luo Xiaozhou are the corresponding authors of the paper.
Screenshot of the published paper
Link of the paper: https://doi.org/10.1016/j.ymben.2023.04.011
The use of cheap carbon sources such as agricultural waste to produce value-added chemicals such as bioenergy, natural products, and bulk chemicals is an industrial production strategy that helps achieve “carbon neutrality”. Polysaccharides such as cellulose and starch are important components, but due to the efficiency limitation of the bio-utilization process, they often cannot be fully utilized. As a conventional model eukaryote, Saccharomyces cerevisiae is often used to secrete or surface-display proteins with different functions. In particular, its extracellularly expressed polysaccharide hydrolase such as cellulase and amylase can convert these cheap carbon sources into high value-added products through the Consolidated Bioprocess (CBP). Modifying the secretory pathway of Saccharomyces cerevisiae to increase extracellular yield of recombinant proteins is one of the well-known schemes. The biosynthesis of cell walls closely regulates the protein secretion pathway, however, its impact on the extracellular expression activity of recombinant proteins has been less reported.
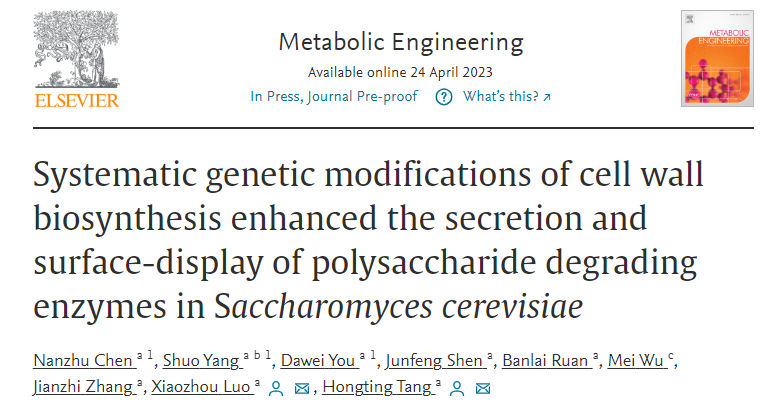
In this study, the author first selected the β-glucosidase (BGL1) as the reporter protein, and its secretion (sBGL1) and surface-display (dBGL1) activities as indexes to systematically evaluate the impact of knocking out 79 genes involved in cell wall synthesis on the activities of sBGL1 and dBGL1. After culture and screening by 96-deep well plate and secondary validation by shake flask fermentation, it was found that the inactivation of DFG5, YPK1, FYV5, and CCW12 or KRE1 genes significantly increased the activity of sBGL1 or dBGL1 (>40%) (Figure 1).
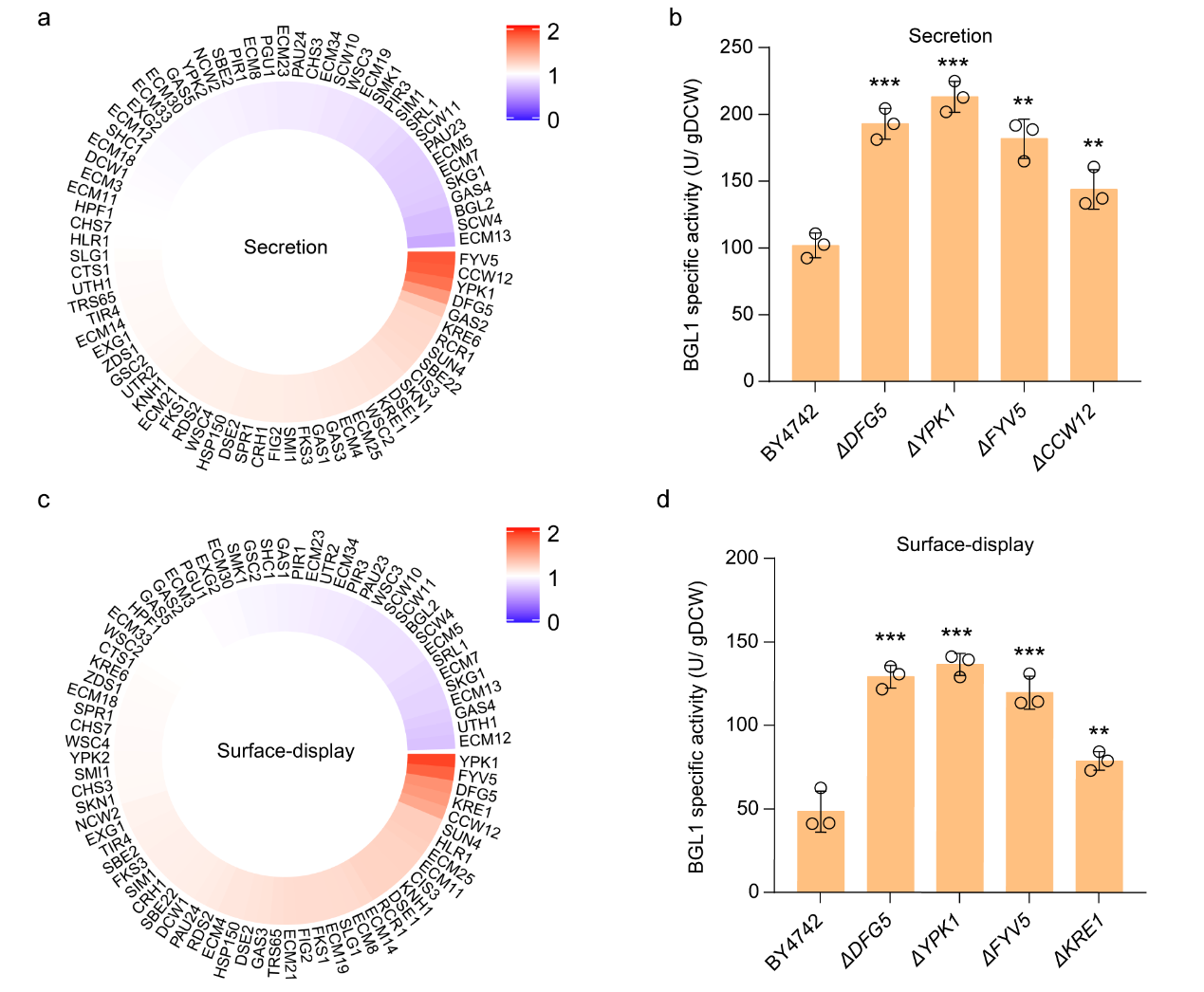
Figure 1. Impact of inactivation of cell wall biosynthesis genes on BGL1 activity.
Subsequently, in order to further explore whether there is a combinatorial effect in the modification of the above genes, the study conducted combinatorial knockouts on the above gene targets, including double knockout, triple knockout, and quadruple knockout. By analyzing their effects on secretion and surface-display of BGL1 activity, it was found that the FYV5 and CCW12 double knockout strains exhibited the optimal extracellular expression ability of BGL1, and their sBGL1 and dBGL1 activities increased by 4.41 and 5.14 times compared with wild-type strains, respectively (Figure 2).
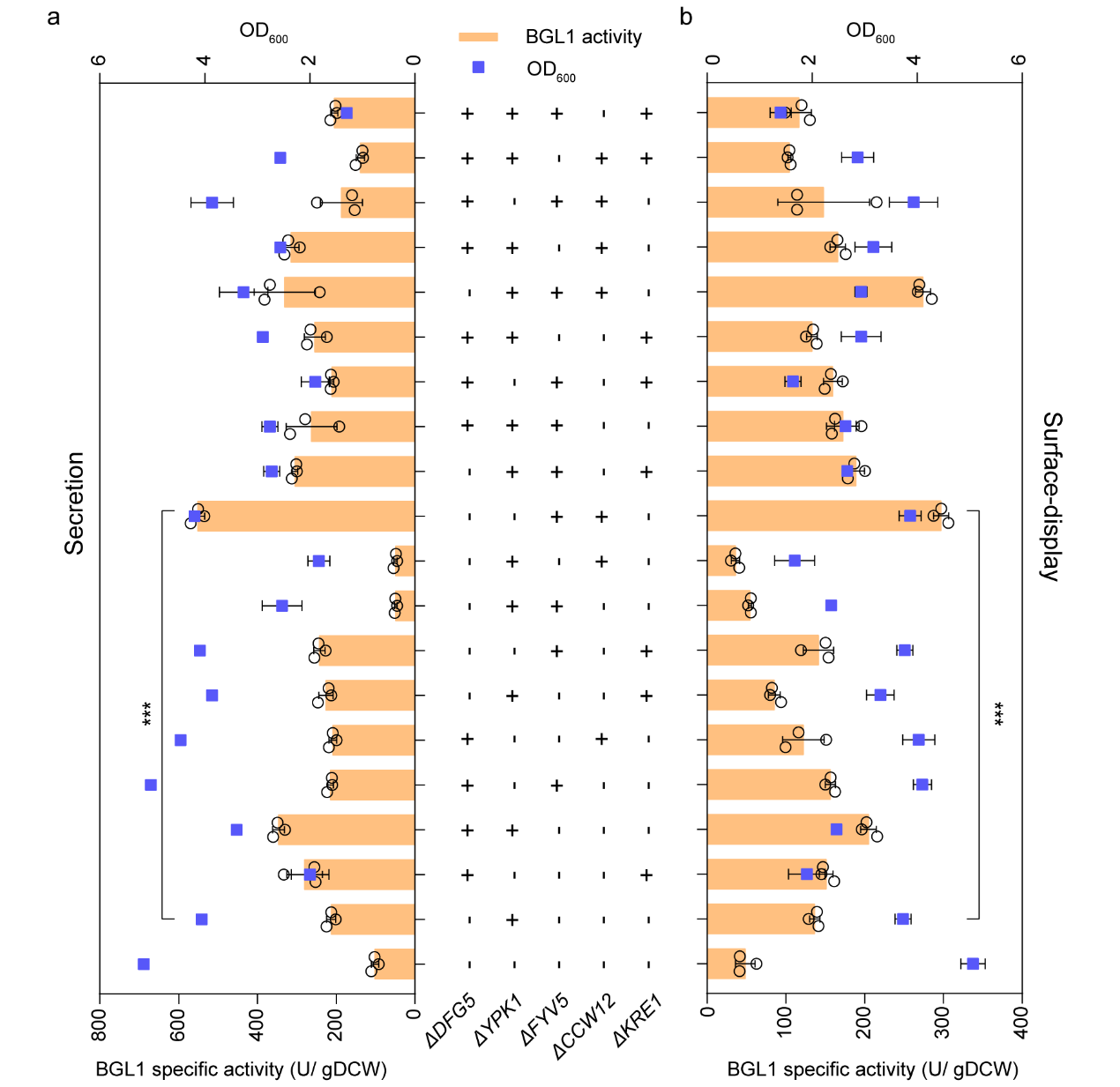
Figure 2. Combinatorial modification of cell wall biosynthesis further enhanced the activity of sBGL1 (a) and dBGL1 (b)
Generally, eutrophic medium YP can promote extracellular expression of proteins. Therefore, the study also tested the changes in extracellular expression activity of recombinase in FYV5 and CCW12 double knockout strains under YP conditions. The results showed that the activity of sBGL1 and dBGL1 in YP increased by 31.68% and 46.55%, respectively.
Figure 3. Eutrophic medium YP further enhanced the secretion and surface-display of BGL1 activity of the strains ΔFYV5ΔCCW12.
To verify the universality of the modified cell wall synthesis pathway, the study tested the impact of FYV5 and CCW12 double knockout on different ankyrin (Sed1p, Dan4p, Aga1p) and different recombinase. The findings showed that the double knockout of FYV5 and CCW12 could improve the activity of BGL1 using different ankyrin, and also promote the activity of different recombinase using the same ankyrin (Figure 4), including cellobiohydrolase (CBH1) and amylase.
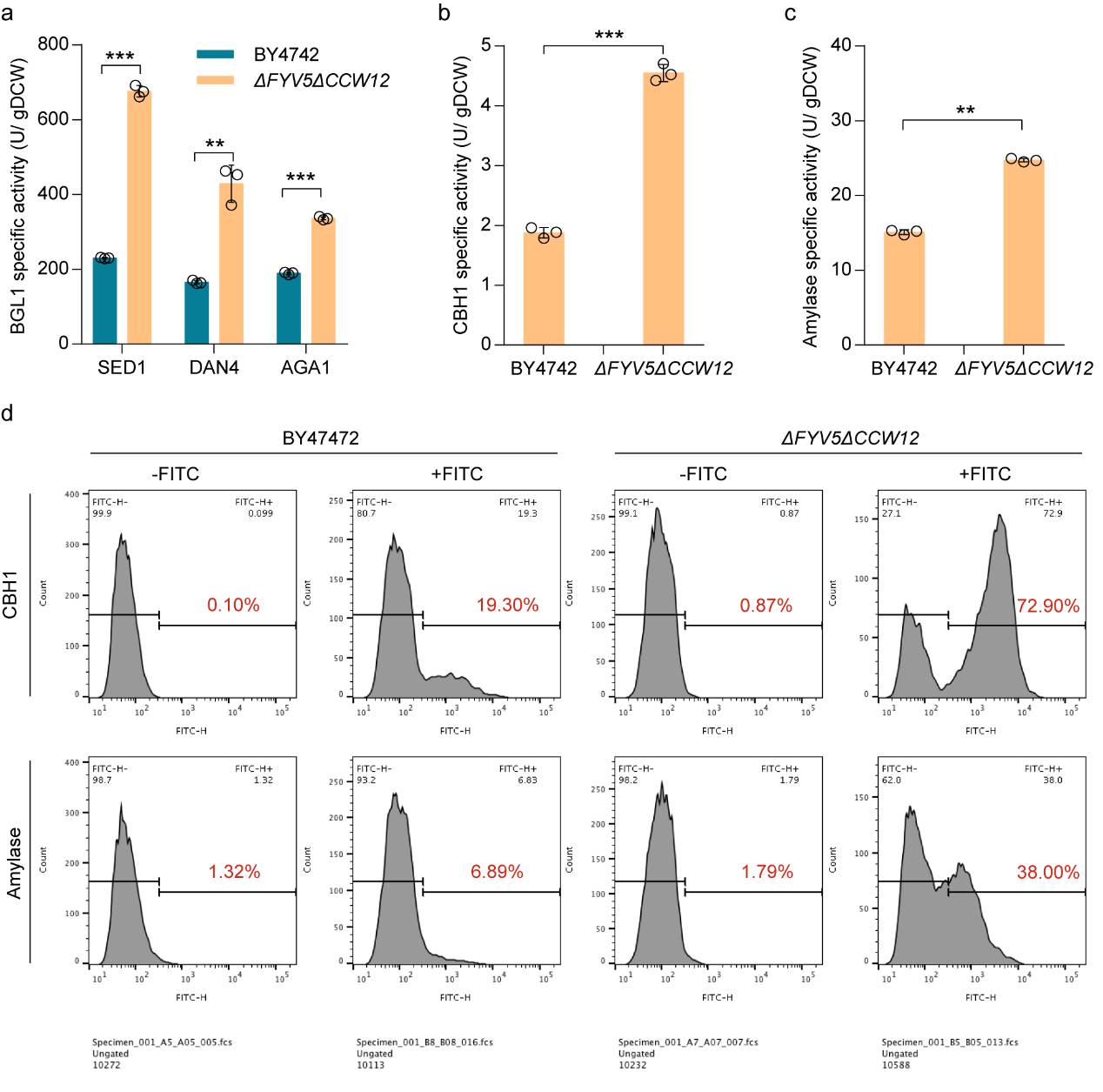
Figure 4. Strains after cell wall biosynthesis modification improved the activity of different recombinase
To explore the potential mechanism by which FYV5 and CCW12 double knockout strains improved extracellular expression of recombinase, proteomic tests were performed on both modified and wild-type strains in the study. It was found that the double knockout of FYV5 and CCW12 could induce the up-regulated expression of a series of proteins. In addition to the key node of the secretory pathway - protein processing in the ER, protein translation process is also one of the main regulated modules (Figure 5). Through reverse metabolic engineering research, it was found that overexpression of 12 proteins in the protein translation process could improve the extracellular activity of BGL1. In particular, overexpression of RPL8B, DBP1, TIF3, ETT1, and SUI3 increased BGL1 activity by 6.8, 2.7, 1.7, 1.2, and 1.1 times, respectively (Figure 5c).
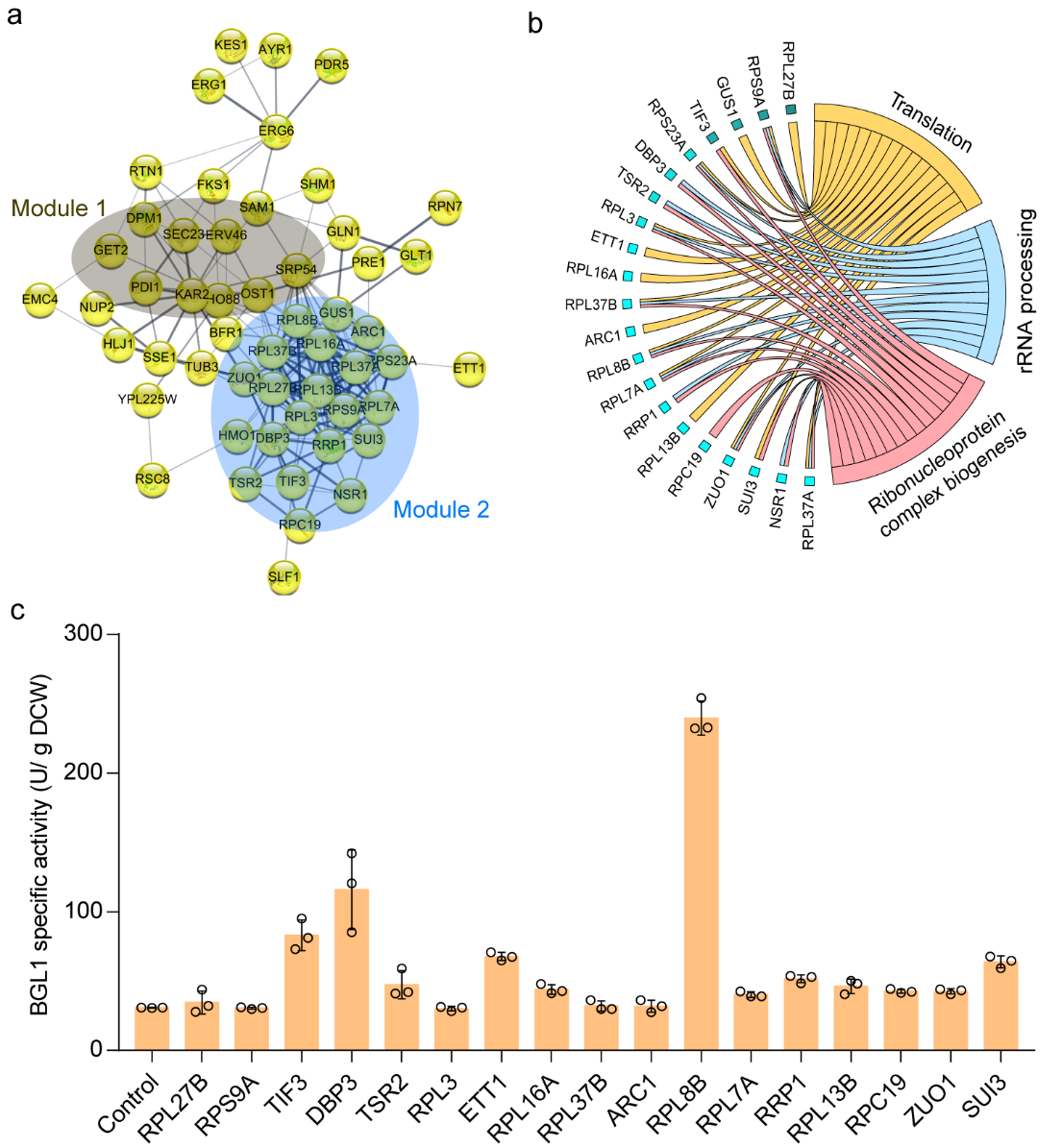
Figure 5. Reveal the mechanism of modifying cell wall biosynthesis pathways to improve recombinase activity through proteomic analysis and reverse metabolic engineering.
In summary, researchers systematically explored the impact of cell wall biosynthetic pathways on the extracellular expression of recombinant proteins in Saccharomyces cerevisiae, and found that the modification of several new targets could significantly increase the extracellular activity of recombinase, including DFG5, YPK1, FYV5, CCW12, and KRE1. Through the combinatorial modification of specific genes and the optimization of medium, the secretion and display activity of extracellularly expressed recombinase in Saccharomyces cerevisiae were greatly enhanced, which increased by 6.13 times and 7.99 times, respectively. Finally, through proteomic analysis coupled with reverse engineering strategies, it was revealed that the protein translation process is one of the important regulations for efficiently expressing recombinase in modified strains. This study provides a new idea for efficiently constructing yeast cell factories.
Acknowledgement
This work was supported by the National Natural Science Foundation (31901030), the Guangdong Basic and Applied Basic Research Foundation (2021A1515010842), and Shenzhen Institute of Synthetic Biology. The researchers also expressed their sincere gratitude to Dai Junbiao, a visiting researcher of the Synthetic Genomics Center of Shenzhen Institute of Advanced Technology, who provided the yeast knockout strain library.