
On April 13, Si Tong's research group of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, published online the research paper in ACS Catalysis, an international academic journal, under the title of “RhlA Exhibits Dual Thioesterase and Acyltransferase Activities during Rhamnolipid Biosynthesis”. Rhamnolipid (RL) is one of the most promising biosurfactants. RhlA enzyme is a key factor affecting the structure of RL products, but its sequence - structure - function relationship is yet unclear, and the catalytic mechanism is controversial. The research team relied on the major technological infrastructure of synthetic biology research in Shenzhen to analyze the protein crystal structure of Rhla for the first time. They unexpectedly discovered that free 3-hydroxy fatty acids can serve as intermediates in the RL biosynthetic process, demonstrating that RhlA had dual thioesterase and acyltransferase activities. By combining semi-rational design and high-throughput screening by mass spectrometry, the team obtained RhlA mutants with significantly altered substrate selectivity. This study provides a mechanism basis and technical reference for the efficient and specific industrial production of rhamnolipid products in the future. The first author of the paper is Dr. Tang Ting, an assistant researcher in the research group, and the corresponding authors are researcher Si Tong and assistant researcher Zhang Jianzhi.

Screenshot of the published paper
Link of the paper: https://pubs.acs.org/doi/10.1021/acscatal.3c00046
Biosurfactants are surface active molecules produced by animals, plants, and microorganisms; compared to chemically synthesized surfactants, they have more diverse structures and good environmental compatibility. Therefore, biosurfactants can serve as green alternative raw materials for important chemical products such as detergent, emulsifier, wetting agent, foaming agent, and dispersants. Rhamnolipid has the ability to significantly reduce the surface/interface tension between mobile phases, and exhibits excellent foaming, emulsification, and flocculation. It also has the advantages of stability, low toxicity, biodegradability, etc. Therefore, it has broad application prospects in fields such as petroleum, environment, agriculture, food, and medicine. The bio-based rhamnolipid products developed by Jeneil, the United States, was awarded the “Presidential Green Chemistry Challenge Award” by the U.S. Environmental Protection Agency in 2004.
Nevertheless, the large-scale application of rhamnolipid is still subject to high production costs, of which about 70% are separation costs; one important reason is that there are a large number of homologues with similar structure and difficult to separate in the biosynthetic rhamnolipid. Intensive study and modification of the biosynthesis pathway of rhamnolipid will help achieve efficient and specific production of target homologues, thereby reducing separation costs, promoting industrial production and market promotion, and realizing green substitution for synthetic surfactants.
RhlA is a key factor affecting the diversity of the molecular structures of RL. RhlA catalyzes the synthesis of 3-hydroxyalkanoyl-3-hydroxyalkanoic acid (HAA), the hydrophobic module of RL. The substrate selectivity of this enzyme determines the length and unsaturation of the aliphatic chain in the hydrophobic module, which in turn affects the physicochemical properties and biological activity of RL. However, due to the limitation of experimental means, the mechanism analysis and engineering modification of RhlA substrate selectivity are still in the initial stage. For example, up to now, no studies have been reported on the crystal structure of Rhla protein, and semi-rational design guided by homologous structure modeling has not effectively altered the substrate selectivity of Rhla. In addition, there are only fine molecular structure differences between rhamnolipid homologues, and qualitative and quantitative analysis relies on methods such as chromatography-mass spectrometry. Their low analysis speed (30 minutes/sample) also limits the application of directed evolution strategies.
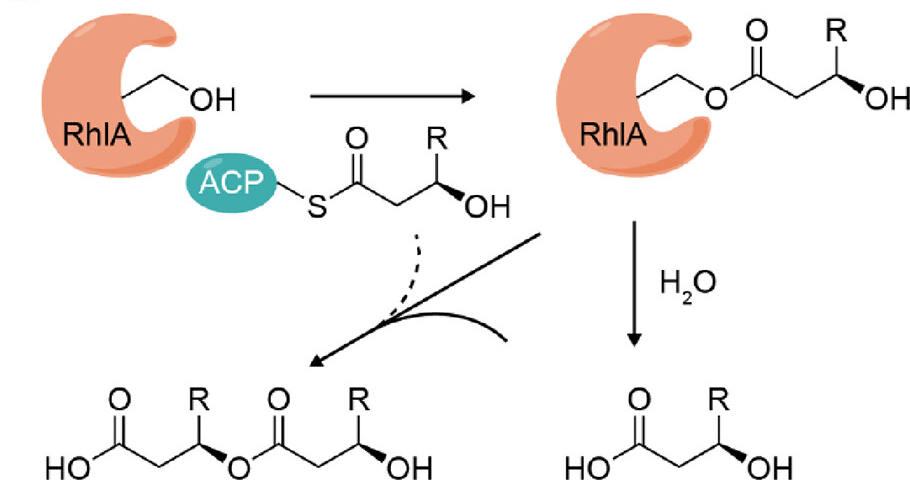
To overcome these limitations, in this study, the protein structure of RhlA from Pseudomonas aeruginosa was first analyzed by X-ray crystallography for the first time, revealing that RhlA has α/β hydrolase folding structure. Surprisingly, in addition to the classical ligand binding pocket 1 near the Ser-His-Asp catalytic center, researchers also found another ligand binding pocket 2, which contains free 3-hydroxy fatty acid and is speculated to be a reaction intermediate.

By combining isotope labeling, in vitro characterization, and QM/MM computational simulation means, researchers verified that RhlA had thioesterase activity in vitro reactions and could catalyze the hydrolysis of acyl-ACP (acyl carrier protein) substrate to produce free 3-hydroxy fatty acid. At the same time, the team found that free 3-hydroxy fatty acid can also serve as an acyl receptor to form HAA with acyl-ACP donors under the catalysis of RhlA acyltransferase activity, but this pathway contributed little in the whole reaction.
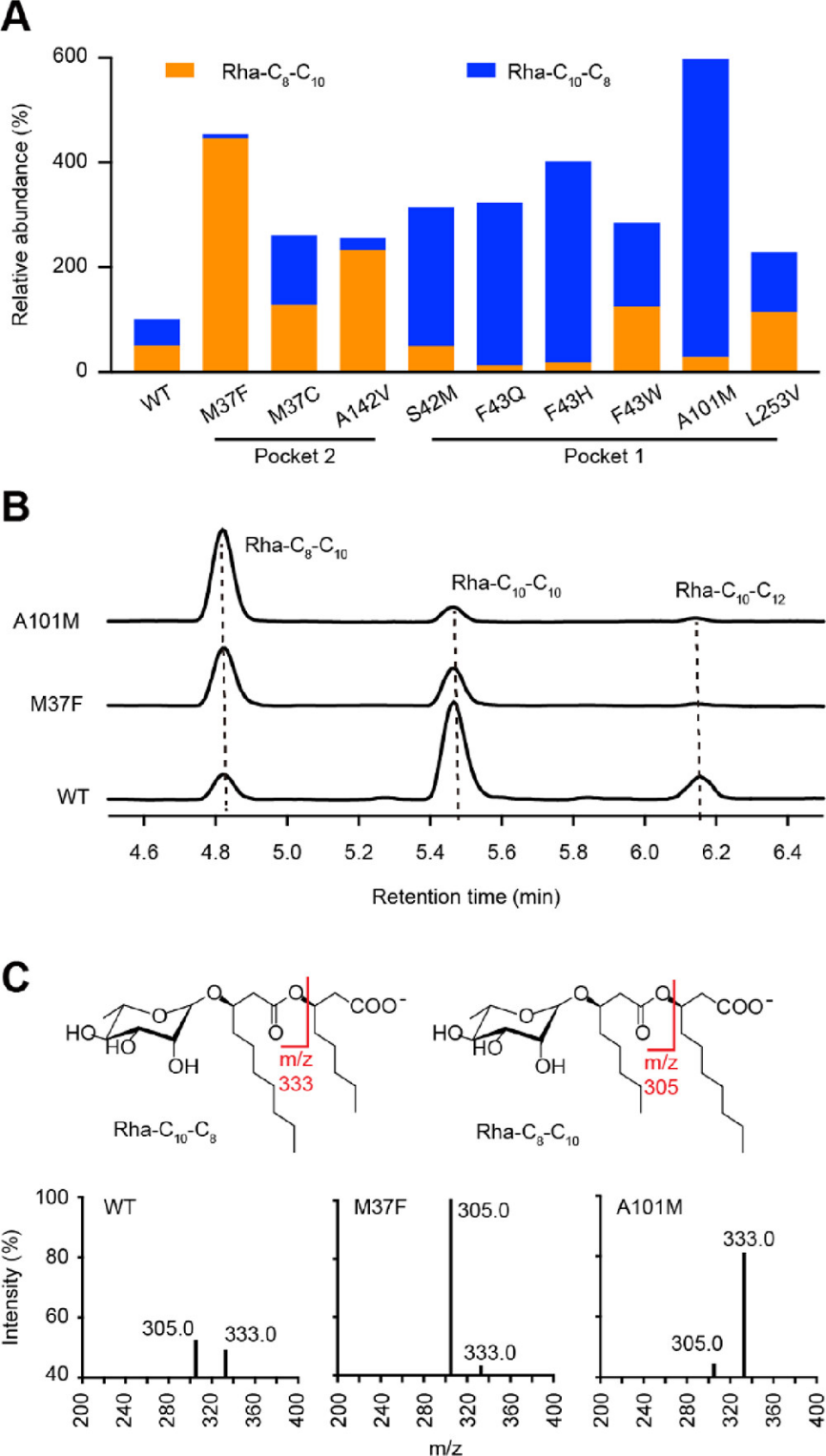
Researchers further applied semi-rational design to construct a locus-specific saturation mutagenesis library targeting 13 key amino acid residue loci in two binding pockets. Thousands of mutant strains were screened by high-throughput MALDI-TOF mass spectrometry, and 9 mutants with significant changes in RhlA substrate selectivity were finally obtained. It was found that mutations of different binding pockets had different regulatory effects on the length of two aliphatic chains in HAA. The team made recombinant expression, protein purification, and in vitro enzyme kinetic analysis on three typical mutants of RhlA (Met37Phe, Phe43His, and Ala101Met), and found that kcat is a key parameter determining the proportion of different RL homologues in the fermentation broth.
It is worth noting that although a possible reaction pathway for RhlA enzyme was analyzed in this study, the catalytic mechanism of RhlA still required further clarification since the cocrystallization structure of RhlA and acyl-ACP substrate had not been obtained.
This achievement was supported by the National Key Research and Development Program (2020YFA090023 and 2021YFA0910800), the National Natural Science Foundation (32071428), the Guangdong Basic and Applied Basic Research Foundation (2022A1515110680), the Strategic Priority Program of the Chinese Academy of Sciences (XDB0480000), Shenzhen Huada Gene Research Institute Development Foundation (BGIRSZ20210003), and Shenzhen Institute of Synthetic Biology. The author particularly thanks Shanghai Synchrotron Radiation Facility and Shenzhen Major Technological Infrastructure for Synthetic Biology Research for their help in X-ray crystal diffraction and high-throughput mass spectrometry screening research.