
In recent years, the rapid development of synthetic biology has brought new opportunities for the deep optimization of tumor bacteriotherapy. Based on synthetic biology methods, scientists can use genetically engineered microorganisms or cells instead of conventional chemical small molecules or biological agents as the basis for developing new therapies for diseases. Artificially designed microorganisms or cells carrying synthetic genetic circuits can respond to disease markers or external signals to realize control of the release location, release time, and release dose of drug, and have gradually developed into a powerful weapon for humans to combat diseases.
On the basis of conventional bacterial modification, rationally designed genetic circuits endow chassis bacteria with more diversified therapeutic abilities, making up for the drawbacks of natural strains in tumor treatment. As a result, tumor bacteriotherapy has become a new tumor therapy with good application prospects. The optimization strategies for existing tumor bacteriotherapy mainly focus on the application of new drugs and the introduction of new controllable release modes, etc., but the impact of bacterial behavior on the therapeutic effect of tumor is ignored, and the continuous control of bacterial behavior during tumor treatment is lacking. Tumor treatment is a long-term process. Controllable and sustained drug release is the key to improving the effect of tumor treatment. Although sustained drug release has been achieved in the field of materials through polymer hydrogels and lipids, it is still a challenge in tumor bacteriotherapy.
Recently, Jinfan's research group of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, published the research achievement in National Science Review, an international academic journal, under the title of “Programming the lifestyles of engineered bacteria for cancer therapy”. Through the design of synthetic biology, the researchers successfully modified Pseudomonas aeruginosa strains into engineered bacteria with therapeutic efficacy for solid tumors. During treatment, the global phenotype of the engineered bacteria could be precisely controlled by the irradiation process of near-infrared light, that is, switching between three phenotypes, namely weak colonization, colonization, and lysis drug release, thereby more effectively ablating the tumor to achieve the therapeutic effect, which has great potential application value. The reviewer believed that “The overall system design is highly elegant”, “I believe the work will have a major impact in the field”. Zhang Rongrong, an assistant researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, is the co-first author and co-corresponding author of the paper, and Researcher Jin Fan is the corresponding author of the paper.

Screenshot of the published paper
Link of the paper: https://doi.org/10.1093/nsr/nwad031
In nature, Pseudomonas aeruginosa is planktonic in favorable environments, and its ability to colonize the surface is weak; in unfavorable environments, it will enter the biofilm state, and its surface colonization ability is greatly enhanced. Inspired by the survival mode of bacteria in nature, the research team designed planktonic and biofilm states for engineered bacteria to control their colonization ability. In particular, planktonic bacteria have weak colonization ability and can reduce damage to normal tissues; while bacteria in the biofilm state have strong colonization ability, which can increase their colonization in tumor tissue. The research team constructed attenuated Pseudomonas aeruginosa strains as novel chassis strains in tumor bacteriotherapy by knocking out the virulence factor regulatory protein coding gene vfr and the Type III secretion system related genes exos and exoT. The switching between the planktonic and biofilm states of Pseudomonas aeruginosa is closely related to the concentration of c-di-GMP, the second messenger molecule. To this end, the research team used two gene modules to control the concentration of intracellular c-di-GMP in bacteria. They were: 1) Phosphodiesterase PA2133 was expressed through a constitutive promoter to decompose c-di-GMP, so that the bacteria maintained a low intracellular c-di-GMP level and entered a planktonic state; 2) The photosensitive protein BphS that can synthesize c-di-GMP under near-infrared light irradiation was introduced. Near-infrared light irradiation could increase the intracellular c-di-GMP level of bacteria, and the bacteria entered the biofilm state. In addition, in order to realize controlled release of the therapeutic drug, the research team designed a third lifestyle for the engineered bacteria, namely the lytic state. By expressing the lytic gene through the c-di-GMP responsive promoter, bacteria could enter the lytic state after c-di-GMP increased (Figure 1). In this way, the three lifestyles of bacteria were all correlated with the concentration of c-di-GMP. By changing the irradiation intensity of near-infrared light, the quantity of activated BphS protein can be controlled, and then the concentration of intracellular c-di-GMP in the engineered bacteria was regulated, thus the control of bacterial lifestyle was realized. In addition to the impact of light intensity, the results of theoretical simulations showed that the intensity of the ribosome binding site (RBS1) upstream of PA2133 and the ribosome binding site (RBS2) upstream of anti-termination protein Q would have a great impact on the near-infrared light intensity required for the bacteria to enter different lifestyles.

Figure 1 Design of genetic circuits for programming bacterial lifestyles.
In order to obtain engineered bacteria that can present three lifestyles under irradiation of different intensities of near-infrared light, the research team replaced RBS1 and RBS2 in batches, screened candidate strains in batches with a 96-well light device, and then verified them with a microscope. The resulting engineered strain was named H017 (Figure 2). The lifestyle of H017 can be programmed by hierarchically regulating the irradiation intensity of near-infrared light. When applying a moderate - high intensity cycle of near-infrared light irradiation process to H017, the bacteria would enter the biofilm - lysis state lifestyle cycle.

Figure 2 Programm bacterial lifestyle by hierarchically regulating the intensity of near-infrared light.
To verify the role of the three lifestyles of engineered bacteria in tumor treatment in vitro, the research team conducted bacteria - cell co-culture experiments in microfluidic channels (Figure 3). The research team first demonstrated that when different intensities of near-infrared light were applied to cells in a designated area, the engineered bacteria could precisely enter the corresponding lifestyle in that area. When a moderate - high intensity irradiation program was applied to cells in a designated area, H017 first entered the biofilm state and colonized the cell surface in large quantities, then entered the lysis state to release the therapeutic drug to cause cell necrosis. The above results showed that the lifestyle of engineered bacteria could be controlled with high temporal and spatial resolution by near-infrared light. In addition, based on the results achieved by the control group, the research team found that the drug accumulation process in the biofilm state and the drug release process in the lysis state were crucial for H017 to kill tumor cells.

Figure 3 Program the lifestyle transformation of “biofilm - lysis” of bacteria to realized controlled release of dugs.
Subsequently, the research team used a mouse subcutaneous tumor model to explore whether the lifestyle of engineered bacteria within the tumor could be controlled by near-infrared light and the expected functions could be realized (Figure 4). The results showed that after bacteria were injected into the tumor, there was no significant difference in the quantity of bacteria in the tumor tissue of the mice cultured in dark for three days (D3) from the group injected with PBS, indicating that bacteria entering the planktonic lifestyle were difficult to colonize in the tumor; the quantity of bacteria in the tumor tissue of the mice in the group irradiated with moderate intensity of light for three days (M3) significantly increased compared with Group D3, indicating that the colonization ability of bacteria entering the biofilm state lifestyle in the tumor tissue was greatly enhanced; compared with Group M3, the mice in the group irradiated with moderate intensity of light for two days and high intensity of light for one day (M2-H1) had a significantly decreased quantity of bacteria, and the tumor tissue slices of Group M2-H1 showed obvious necrotic areas, indicating that the bacteria released the drug and killed tumor cells after lysis; compared with Group M2-H1, the mice in the group irradiated with moderate intensity of light for two days, then high intensity of light for one day, and moderate intensity of light for two more days (M2-H1-M2) showed increased quantity of bacteria again, indicating that the bacteria entered a state of colonized growth again after being irradiated by moderate intensity of light. In addition, after seven days of irradiation with moderate intensity of near-infrared light, the engineered bacteria in the tumor still had the ability to lyse, indicating that the genetic circuit had good stability. Based on the above results, it can be concluded that irradiation with different intensities of near-infrared light can make the bacteria in the tumor enter the corresponding lifestyle and achieve the expected functions, and the continuously changed irradiation process can achieve continuous control of the lifestyle of the bacteria in the tumor.
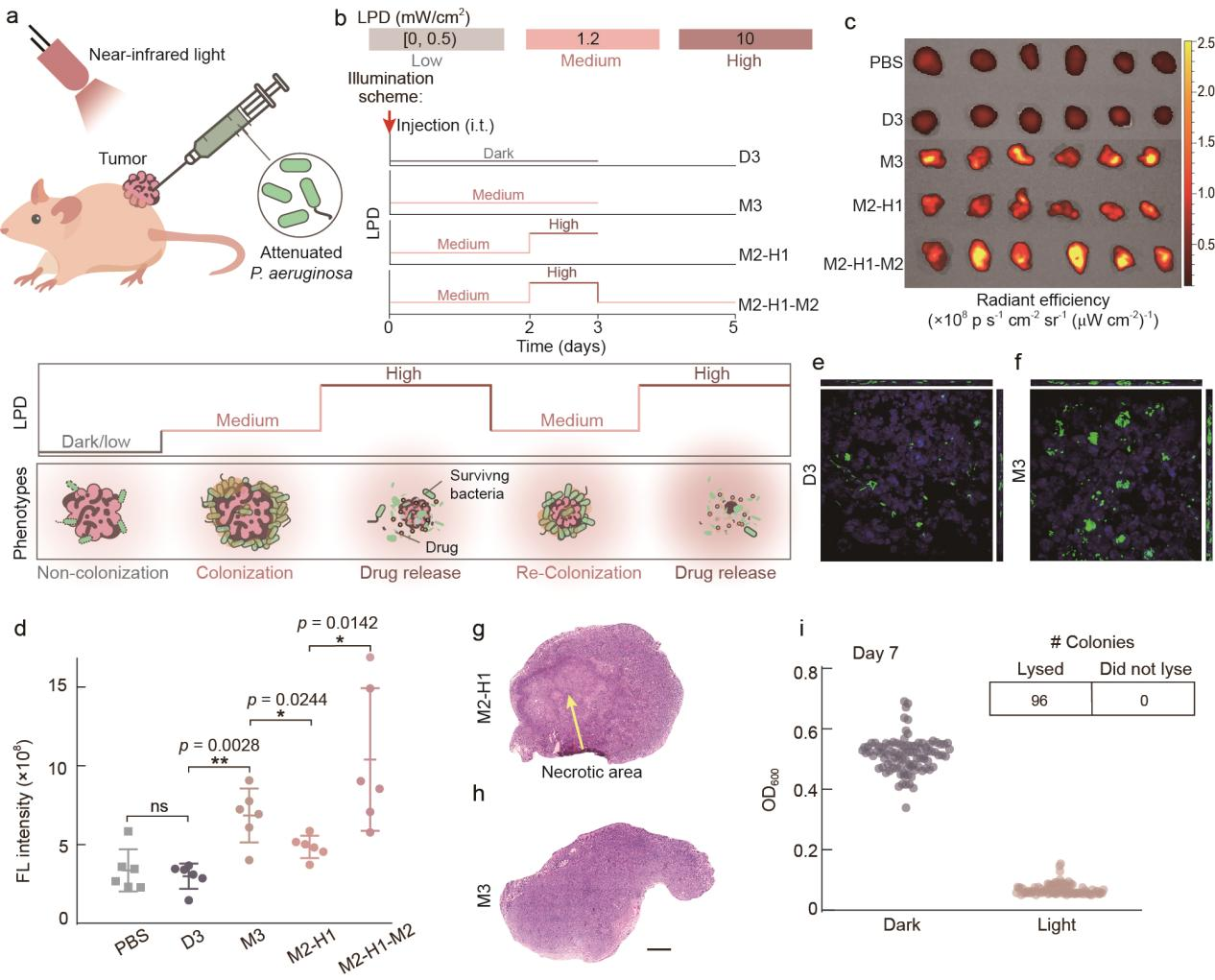
Figure 4 Control the bacterial lifestyle in solid tumors by near-infrared light.
Finally, the research team explored whether the tumor treatment effect of engineered bacteria can be enhanced by programming its lifestyle (Figure 5). When H017 was only used as a drug delivery carrier, high intensity of near-infrared light could make H017 lyse and release the drug to complete tumor treatment. The team effectively inhibited tumor growth by injecting bacteria 8 times during a 20- day experimental period. Next, the research team controlled the lifestyle of bacteria through the irradiation process to achieve continuous control of drug accumulation and release. The results showed that after two cycles of a near-infrared light irradiation process including 2-day moderate intensity irradiation - 1-day high intensity irradiation (M2-H1×2) were applied to mice, tumor growth in mice was inhibited, while tumor growth in mice of the control group cannot be inhibited. In a longer cycle experiment (M2-H1×6), tumor growth in all mice was inhibited through tumor treatment by programming the bacterial lifestyle based on irradiation time, with 30% of the tumors in the mice disappeared completely, while the tumors in the mice of the control group continued to grow. The above results indicated that programming bacterial lifestyle had significant advantages in the long-term treatment of tumors, and can achieve better tumor inhibition effect with fewer times of bacteria injection.

Figure 5: Program the bacterial lifestyle in the tumor promoted solid tumor regression.
The research was supported by several programs such as the Key Research and Development Program of the Ministry of Science and Technology of China, the National Natural Science Foundation, the Research Instrument and Equipment Development Program of the Chinese Academy of Sciences, the China Postdoctoral Science Foundation, Chang'an Capital, and Shenzhen Institute of Synthetic Biology, and was greatly assisted by the team of Major Technological Infrastructure for Synthetic Biology.