
On March 30, the latest achievement of the team of Wei Ping, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, was published online in iScience under the title of “Design of Diversified Chimeric Antigen Receptors Through Rational Module Recombination”. In this work, a series of novel chimeric antigen receptors were designed through module recombination, which provided a new toolkit and design idea for improving tumor immunotherapy.

Screenshot of the published paper
Link of the paper: https://doi.org/10.1016/j.isci.2023.106529
Chimeric Antigen Receptor-T cell therapy (CAR-T cell therapy), as a relatively new immunotherapy regimen in recent years, has made many breakthroughs in the field of cancer treatment. The difference of co-stimulatory domains contained in CAR molecules will bring different background and post activation signals to T cells, and then have different effects on the function and fate of T cells. However, current studies lack the understanding of this problem, and the types and quantities of available co-stimulatory domains are extremely limited, resulting in CAR-T cell therapy being unable to meet the diversified demands of clinical practice, and to some extent limiting the effectiveness and safety of this therapy.
The research team defined functional modules from natural co-stimulatory receptors by means of synthetic biology, and designed a series of novel co-stimulatory domains through protein module recombination, thus obtaining a large number of chimeric antigen receptors. Through the dual-reporting system of fluorescent protein-based NFAT (Nuclear Factor of Activated T cells) and NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) signal pathways constructed in Jurkat cell line, a high-throughput platform for quantitatively characterizing T cell signaling characteristics was established, and an effective technical means for identifying and predicting the activity of chimeric antigen receptors was provided from the perspective of signal pathway and mechanism of action. At the same time, most of the chimeric antigen receptors obtained through artificial recombination in this project had the activity of activating T cells, and the response of NFAT and NF-κB signal pathways differed greatly, which greatly expanded the range of activation intensity of chimeric antigen receptors on T cells. In the study, it was found that the activity of NFAT and NF-κB signal pathways was correlated with the function and fate of CAR-T cells..
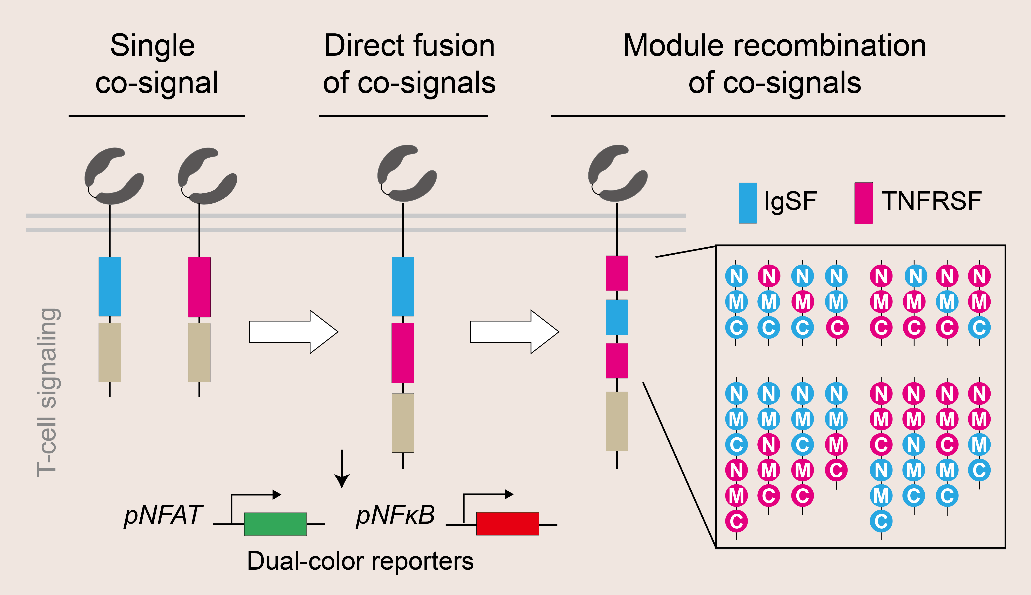
Figure 1 Design and proof-of-concept platform of chimeric antigen receptor composed of recombinant co-stimulatory molecules
The results of further study showed that the recombined 28(NM)-BB(MC) CAR had a signal activation mode that combined the characteristics of CD28 and 4-1BB, showing low background activation and high response intensity. The researchers further stated the impact of this signal characteristic on the function and fate of T cells, and applied it to anti-CD19 (α-CD19) and anti-mesothelin (α-meso) CAR-T cells. Through in vitro experiments at the cellular level, they demonstrated that this type of cell, on the basis of its specific ability to kill target cells, had obtained a similar function of inhibiting cell depletion as 4-1BB, and also showed the characteristics of reducing T cell apoptosis and cytokine secretion. The findings by transcriptomics further reveal the advantages of 28(NM)-BB(MC) in promoting the killing function and inhibiting depletion and apoptosis of T cells. These results suggested that the recombinant co-stimulatory domain may enhance the safety and effectiveness of CAR-T cell therapy.
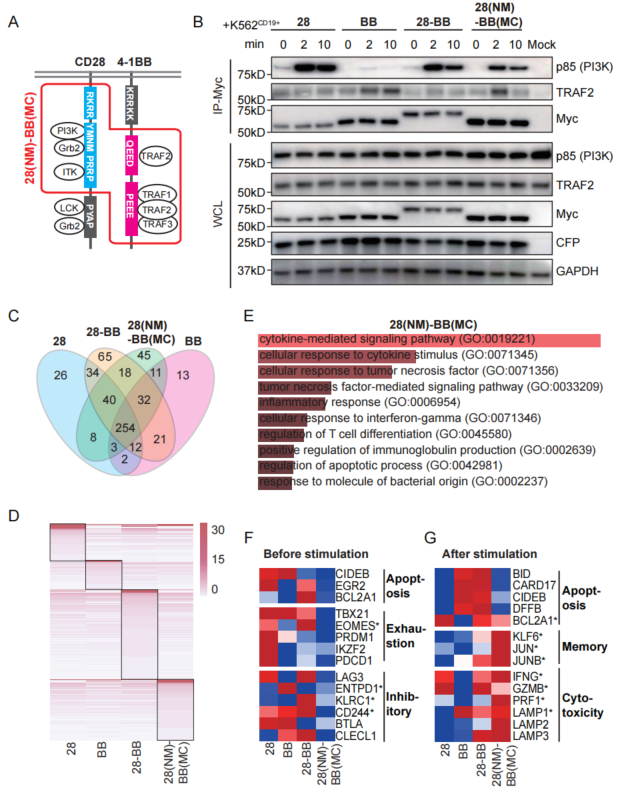
Figure 2 Recombinant 28(NM)-BB(MC) CAR had the activation modes of the co-stimulatory signal receptors 4-1BB and CD28, and had the advantages of balancing the killing function of T cells and inhibiting depletion and apoptosis
This study not only provides a powerful high-throughput quantitative screening platform for the development of a new generation of CAR-T cells, but also provides a new idea for designing CAR by the developed protein module recombination technology. The large number of novel recombinant co-stimulatory domains obtained greatly expand the range of the regulation of intracellular signals in CAR-T cells, which adapt to different application scenarios and antigen stimulation intensities, and have great clinical application prospects.
Wei Ping, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, is the corresponding author of the paper, and Si Wen, a postdoctoral fellow of Peking University, and Fan Yingying, a postdoctoral fellow of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, are the co-first authors of the paper. This work was supported by programs such as the Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, the Strategic Key Research Program of the Chinese Academy of Sciences, and Shenzhen Institute of Synthetic Biology.