
Cell polarity refers to the asymmetry of cell morphology, structure, and function at the cellular level, which is common in bacteria, archaea, and eukaryotes. Cell polarity determines a number of life processes such as asymmetric division of bacteria, daughter cell fate determination, cell movement, and cell differentiation, and regulates the pathogenicity and the stress resistance to antibiotics of bacteria.
How cell polarity is established and dynamically adjusted over time and space is a core scientific problem in this field. Zhao Guoping - Zhao Wei's team found in preliminary studies that scaffold proteins were necessary for establishing cell polarity: scaffold proteins recruited and organized cell fate determination proteins through phase separation, promoting the construction of new cell poles and the remodeling of old and new cell poles (Nature Communications 13:7181 2022). In the process of new cell pole establishment, the scaffold protein PopZ would shift from old cell pole localization to bipolar localization, which is of great significance for the development of new cell poles and the different signal transduction between old and new poles. However, it has long been unclear how PopZ subcellular localization is regulated.
Recently, Zhao Guoping - Zhao Wei's team of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, published a research achievement in mBio, an international academic journal, under the title of Scaffold-scaffold interaction facilitates cell polarity development in Caulobacter crescentus. In this study, it was found that the scaffold protein PodJ regulated the subcellular localization of PopZ through protein-protein interaction, which determined the emergence time of PopZ at the new cell pole. Inhibiting the PodJ-PopZ interaction would trigger abnormal chromosome segregation mediated by PopZ, leading to uncoupling of chromosome segregation and cell division in the cell cycle.

Figure 1. Screenshot of the published paper
Link of the paper: https://journals.asm.org/doi/10.1128/mbio.03218-22
A key event of cell polarization is the formation of protein complexes at the new cell pole with different composition and biological function from those of the old cell pole. The scaffold proteins PopZ and PodJ have both been shown to be organizers of polar protein complexes in the cells of Caulobacter crescentus. Here, researchers found a direct and conserved interaction between the scaffold proteins PopZ and PodJ, which is an important driving force for PopZ to transition from unipolar localization mode to bipolar localization mode.
The research team highlighted the main regulatory role of PodJ in PopZ bipolar localization by comparing it with other PopZ regulatory proteins (such as ZitP and TipN). Subsequent in vivo and in vitro experiments demonstrated that the structural domain CC4-6 in PodJ is a key region that directly interacts with PopZ. PodJ ensures the timely accumulation of PopZ at the new cell pole and the rigorous inheritance of cell polarity. The disruption of PodJ-PopZ interaction led to abnormalities in PopZ-mediated chromosome segregation, which might lead to uncoupling of chromosome segregation and cell division in the cell cycle (Figure 2).
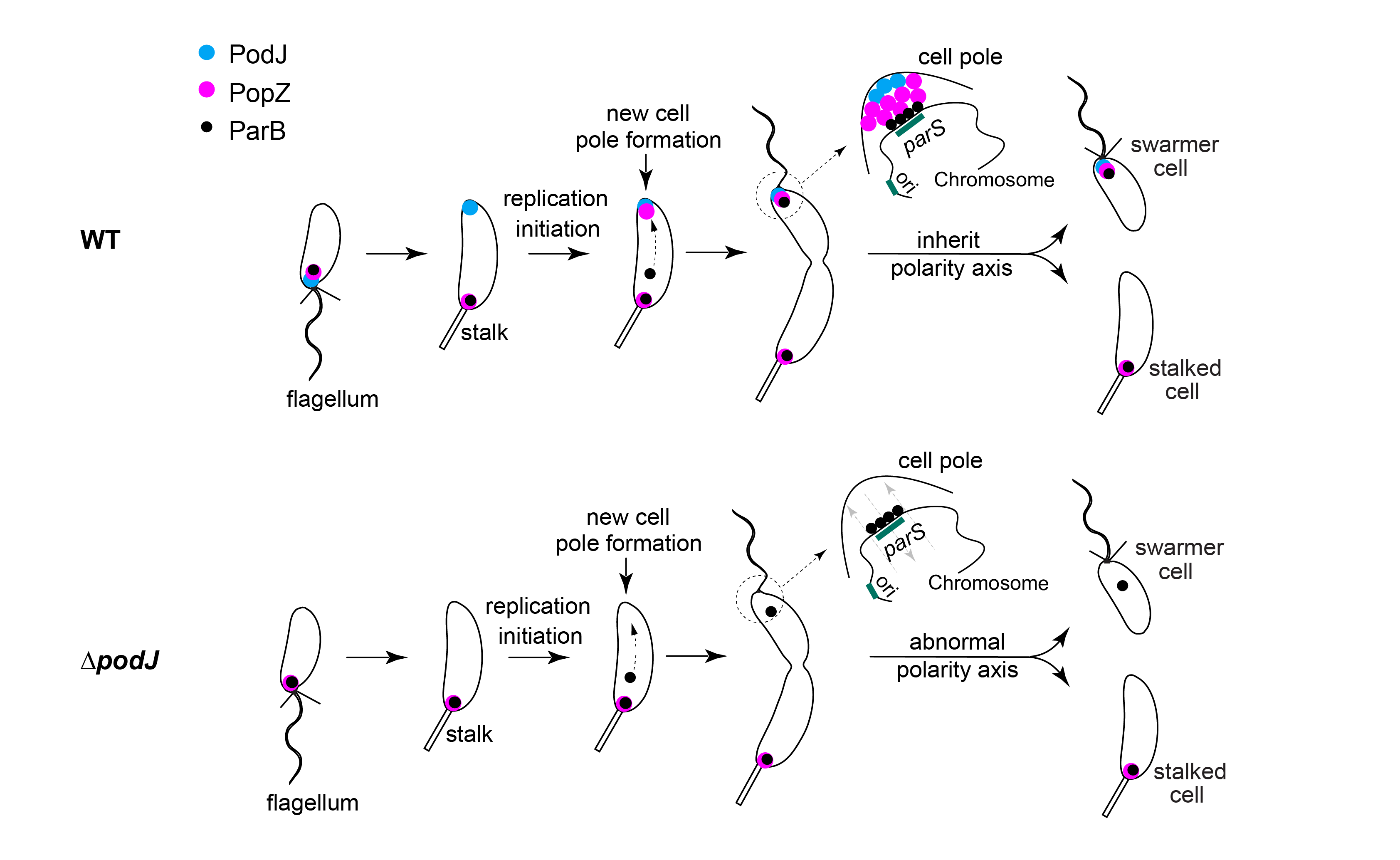
Figure 2. PodJ-PopZ spatiotemporal interaction regulated the cell polarity development of Caulobacter crescentus
This work revealed a dynamic regulatory mechanism that regulates the polarity development of microbial cells through scaffold protein interaction, providing a direction for further exploration of asymmetric cell division. In this study, based on the results of previous work, it was demonstrated that the scaffold protein complex PodJ-PopZ-SpmX constitutes the cell polarity regulatory circuit with unipolar positive feedback and interpolar negative feedback. According to bioinformatics analysis and experimental verification, the PodJ-PopZ-SpmX interaction circuit is common in other α-Proteobacteria, suggesting an evolutionarily conserved cell polarity regulatory mechanism.
Lu Ning, a postdoctoral fellow of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, is the first author of the paper, and Dr. Samuel W. Duvall of the University of Pittsburgh is the co-first author of the paper. Associate Researcher Zhao Wei and Professor W. Seth Childers is the corresponding author of the paper, and Shenzhen Institute of Advanced Technology is the first accomplishment organization and the first corresponding author organization of the paper. The research was sponsored by the National Key Research and Development Program, the Strategic Priority Research Program of the Chinese Academy of Sciences, the Guangdong Basic and Applied Basic Research Foundation Committee, the China Postdoctoral Science Foundation, and the Shenzhen Institute of Synthetic Biology.