
Recently, the latest achievement of the team of Yan Fei, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, was published online in Small (IF: 15.3), a well-known journal in the field of material science, under the title of “Ultrasound Molecular Imaging of Epithelial Mesenchymal Transition for Evaluating Tumor Metastatic Potential via Targeted Biosynthetic Gas Vesicles”. This work used biosynthetic nan’\o-gas vesicles to realize ultrasound molecular imaging of dynamic changes in tumor Epithelial Mesenchymal Transition (EMT), providing a new method for evaluating tumor metastatic potential.
Yan Fei, a researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology is the corresponding author of the paper, and Hao Yongsheng, a master degree candidate of the Institute of Synthetic Biology, Li Zhenzhou, a physician of the Second People's Hospital of Shenzhen, and Luo Jingna, a master degree candidate of Shenzhen University, are the co-first authors of the paper.
Screenshot of the published paper
Epithelial Mesenchymal transition (EMT) is an important factor driving tumor metastasis. Research has shown that the evolution of EMT is accompanied by changes in a variety of molecular markers, among which “CadherinSwitch” is the most typical molecular change: On the surface of tumor cells, the expression of E-cadherin (E-cad) is gradually down-regulated, while the expression of N-cadherin (N-cad) is gradually up-regulated. In this process, tumor cells gradually lose their epithelial features and obtain the mesenchymal phenotype, which enhances the invasion and metastasis ability of tumor cells. Therefore, the detection of tumor EMT changes is of great significance for evaluating tumor metastatic potential. As a medical imaging method with high imaging depth and cost-effectiveness, ultrasound imaging has been widely used in clinical practice. In recent years, nanoscale gas vesicles (GVs) synthesized by microorganisms have provided the possibility for non-invasive in vivo ultrasound molecular imaging detection of tumor cell markers outside tumor vessels.
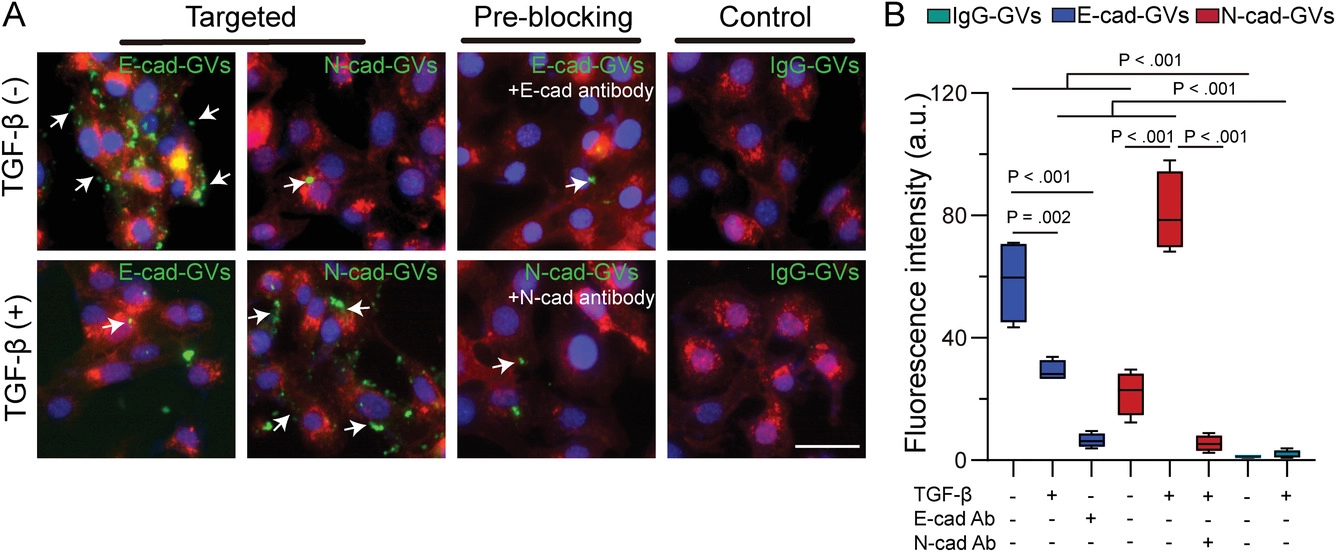
Figure 1. Adhesion effect of E-cad-GVs/N-cad-GVs probes on tumor cells in different EMT states
In this work, the research team first evaluated the tumor vascular permeability of GVs. Compared with micron-sized phospholipid microvesicles, GVs could enter extravascular tumor tissues through the tumor vascular endothelial gap and realize contrast-enhanced ultrasound imaging. Subsequently, the research team used GVs to construct ultrasound molecular imaging probes E-cad-GVs/N-cad-GVs targeting E-cad/N-cad, and tested their ability to adhere to tumor cells in different EMT states. The results showed that the binding efficiency of E-cad-GVs/N-cad-GVs with cells depended on the EMT state of tumor cells (Figure 1).

Figure 2. Results of E-cad-GVs/N-cad-GVs probe imaging of EMT states of early and advanced tumor
To realize in vivo detection of tumor EMT changes, the research team injected E-cad-GVs/N-cad-GVs ultrasound molecular probes into the caudal vein of early and advanced tumor-bearing mice in different EMT states, and performed ultrasound molecular imaging detection on the tumors. The results showed that ultrasound molecular imaging could image different EMT states in early and advanced tumors, and its results were in good agreement with the expression levels of E-cad/N-cad detected by conventional immunohistochemical staining (Pearson r: 0.83), indicating that ultrasound molecular imaging based on E-cad-GVs/N-cad-GVs can be used to evaluate tumor EMT changes (Figure 2). Finally, the research team further analyzed the correlation between the imaging results of tumor EMT changes and tumor metastasis, and further evaluated the feasibility of evaluating tumor metastatic potential based on ultrasound molecular imaging of E-cad-GVs/N-cad-GVs (Figure 3).
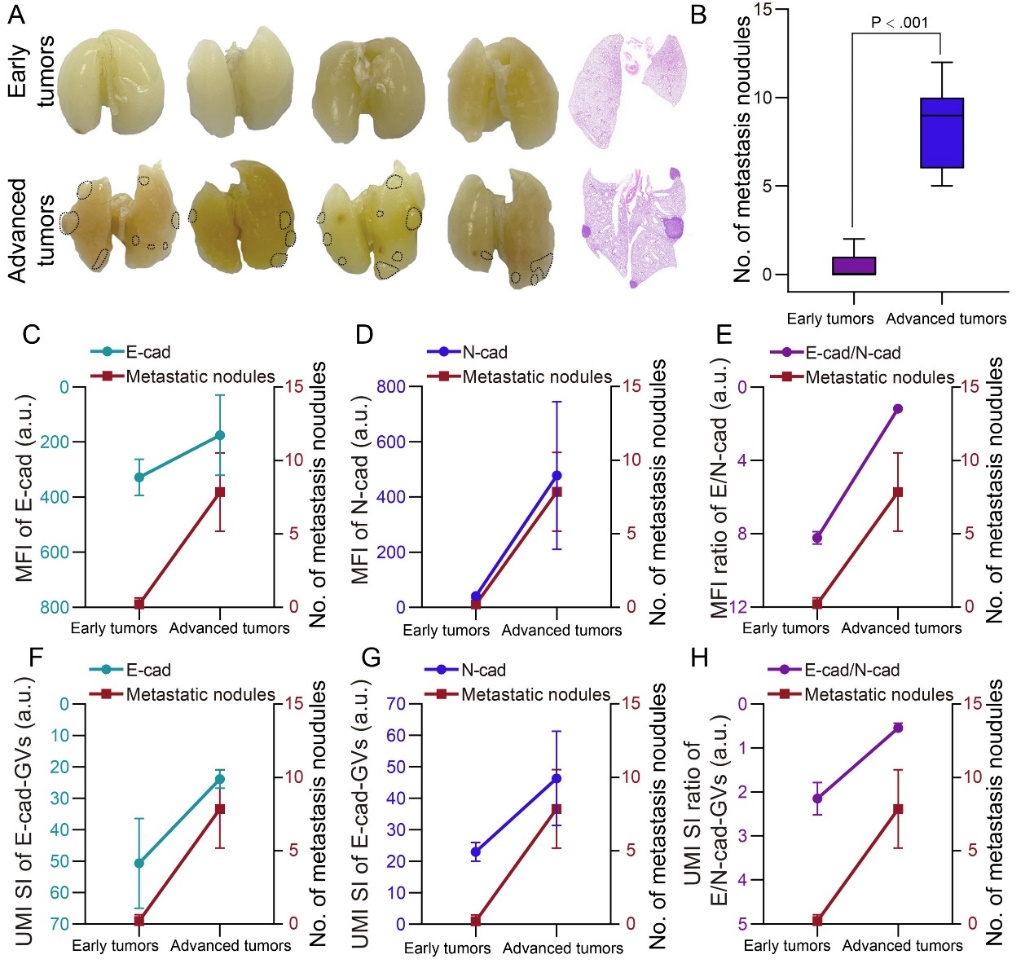
Figure 3. Correlation analysis of ultrasound molecular imaging signals and immunohistochemical signals with tumor lung metastatic nodules
This work was supported by the National Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, and programs of Shenzhen Science and Technology Innovation Committee, Shenzhen Institute of Synthetic Biology, etc.
Hao Y, Li Z, Luo J, Li L, Yan F. Ultrasound Molecular Imaging of Epithelial Mesenchymal Transition for Evaluating Tumor Metastatic Potential via Targeted Biosynthetic Gas Vesicles. Small. 2023; e2207940. doi:10.1002/smll.202207940