
NK cells (Natural Killer cells) are not only direct effector cells in antitumor therapy, but also play an important helper role in antitumor T cell response and T cell-based checkpoint immunotherapy. However, the intrinsic molecular mechanism for NK cells to help T cells is not clear at present.
On February 19, Advanced Science published online the latest research achievement of Academician Tian Zhigang's team, “Checkpoint TIPE2 limits the helper functions of NK cells in supporting antitumor CD8+ T cells,” which reported the molecular mechanism of NK cells in supporting antitumor immune response of CD8+ T cells, and reported that TIPE2 molecules are checkpoint molecules for the helper functions of NK cells, and targeted deletion of TIPE2 in NK cells can simultaneously promote the antitumor response of NK cells and the CD8+T cells they supported. Tian Zhigang and Sun Haoyu, professors of the University of Science and Technology of China, and Bi Jiacheng, an associate researcher of the Institute of Synthetic Biology of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, are the corresponding authors of the paper, and associate researcher Bi Jiacheng is also the first author of the paper.

Screenshot of the published paper
Link of the paper: https://onlinelibrary.wiley.com/doi/10.1002/advs.202207499
The rapid development of immunotherapy is changing the paradigm of tumor treatment. However, not all patients benefit from immunotherapy. In response to solid tumors and other cases, immunotherapy still faces problems such as insufficient effectiveness and safety. On the other hand, under the influence and drive of the concept and technology of synthetic biology, current immunotherapy research is moving towards a new stage of “synthetic immunology” characterized by engineering manipulation. It aims to rationally manipulate the immune response by designing and constructing new synthetic immune molecules, synthetic immune cells, etc., so as to realize the immunotherapy of major diseases such as tumors. The in-depth study of “Immune Checkpoints” (molecules that play an important role in regulating the immune system), an important element in the design of synthetic immune molecules/cells, can not only reveal the regulation law of immune response, but also provide a theoretical basis for the engineering design of synthetic immune molecules/cells. Therefore, the study of “Immune Checkpoints” is the key breakthrough point of the current study of “synthetic immunology”.
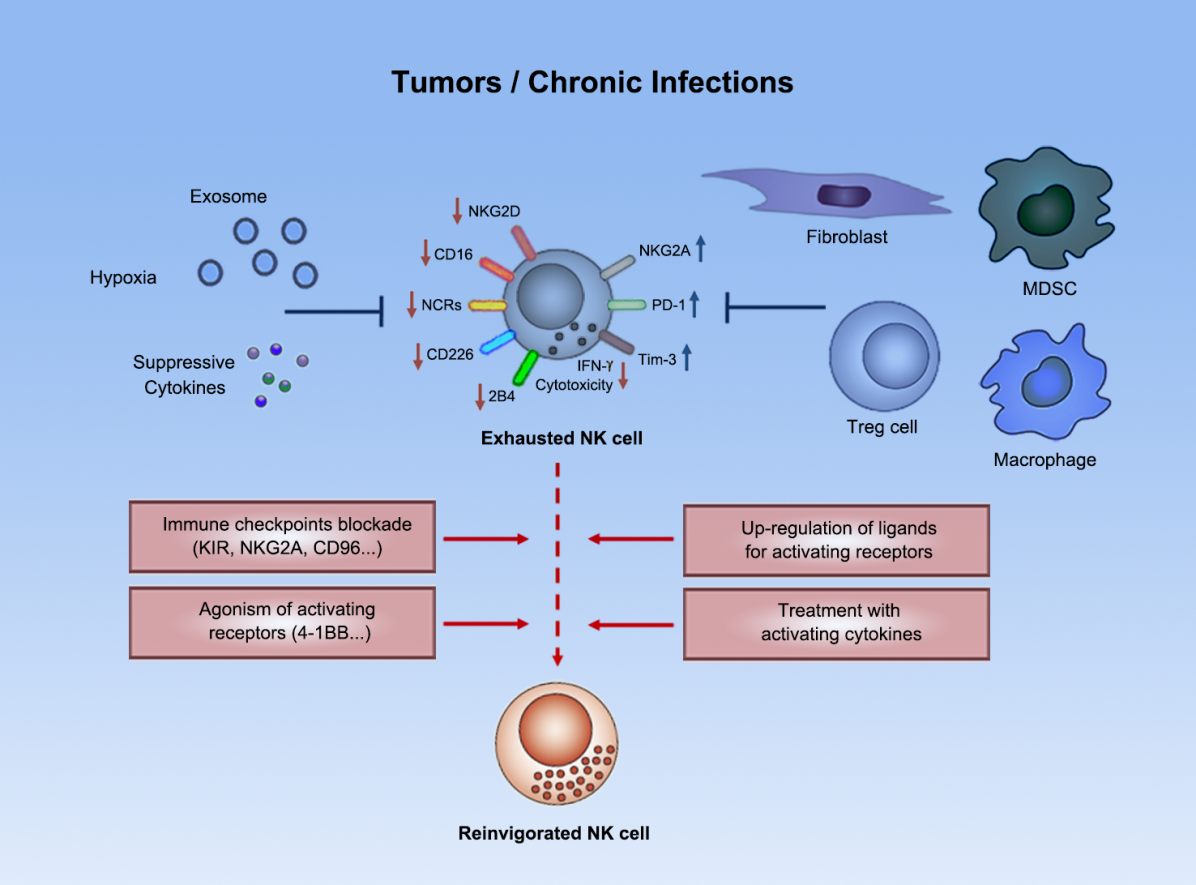
Figure 1. NK cells entered a dysfunctional “immunodepletion” state in tumor microenvironment
NK cells are a kind of innate immune cells that perform immune surveillance on tumors by killing target cells, inducing apoptosis of target cells, or secreting cytokines. A large number of studies have long shown that NK cells not only play a key role in controlling hematological tumors and tumor metastasis, but also their infiltration level in solid tumors is closely related to the prognosis of patients. NK cells play an important role in current tumor immunotherapy research, as they not only recognize and kill tumor cells that escape T cell response such as that have low HLA expression, but also play a role in promoting T cell-based immunotherapy (such as “Immune Checkpoints therapy” targeting PD-1/PD-L1). In addition, NK cells naturally have the advantage of serving as chassis cells in “universal” synthetic immune cell therapy. Nevertheless, NK cells in tumor microenvironment will be negatively regulated by a series of negative immunoregulatory cells, molecules, etc., and enter a dysfunctional “immunodepletion” state (Figure 1) [1,2]; to fully exert the advantages of NK cell-based immunotherapy strategies, researcher teams need to delve into the functional regulatory mechanisms of tumor-related NK cells.
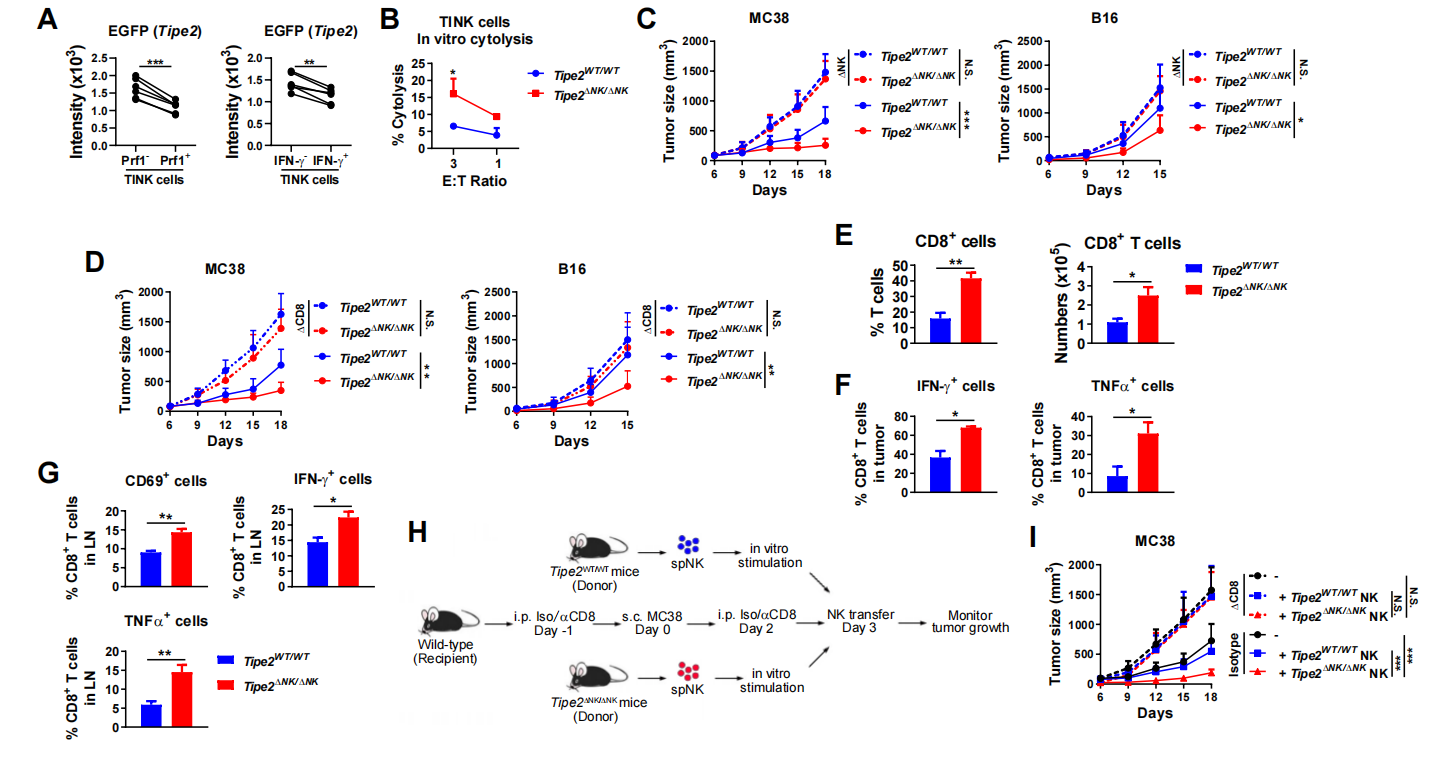
Figure 2. NK cells with Tipe2 depleted promoted antitumor CD8+T cell response
The research group has previously found that the Immune Checkpoints such as TIGIT (Hepatology 2014a, 2014b; Nat Immunol 2018), A3AR (Cell Mol Immunol 2021), and TIPE2 (Science Advances 2021; J ImmunoTher Cancer 2023) had a negative regulatory effect on the function of NK cells in disease states such as tumors, tissue damage, and regeneration[3-8]. The expression level of intracellular Immune Checkpoint TIPE2 in homeostatic NK cells was related to the functional maturation of NK cells, the expression level of TIPE2 in tumor-related NK cells was related to the functional depletion state of NK cells, and the NK cell subsets with high TIPE2 expression was significantly related to poor prognosis of tumor patients.
By using the mouse colon cancer MC38 tumor model and the melanoma B16 tumor model commonly used to study the antitumor response of T cells, the researchers found that NK cells with Tipe2 deleted significantly inhibited tumor growth in mice, which also depended on the presence of both NK cells and CD8+T cells (Figure 2). After Tipe2 in NK cells was deleted, the function of tumor-draining lymph nodes and the CD8+T cells in tumor site was significantly enhanced, and the tumor growth after the NK cells with Tipe2 deleted were transferred to MC38 tumor-bearing mice was significantly lower than that of the control group; if the CD8+T cells were cleared in advance, this effect was not observed. Therefore, the results suggested that the TIPE2 molecules in NK cells indirectly inhibited the antitumor response of CD8+T cells.
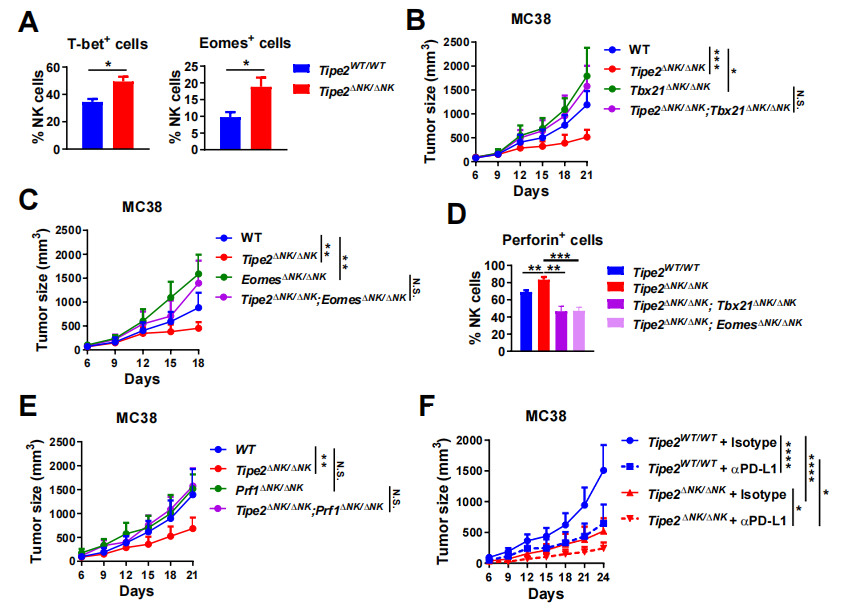
Figure 3. Mechanism of NK cells with Tipe2 deleted promoting antitumor CD8+T cell response
In terms of mechanism of action, as the researchers observed that the tumor-infiltrated NK cells with higher expression of the effector molecules IFN-g and perforin had lower expression of Tipe2, and the tumor-infiltrated NK cells with Tipe2 deleted had stronger killing activity (Figure 2), the research team considered whether TIPE2 indirectly inhibited the effector function of NK cells by inhibiting the upstream molecules of the effector molecules such as IFN-g and perforin, thereby affecting the antitumor response of CD8+T cells. The team found that after the deletion of Tipe2, the tumor-infiltrated NK cells expressed higher levels of transcription factors T-bet and Eomes. The transcription factors T-bet and Eomes promoted the transcription of a series of effector molecules in NK cells, and played a key role in the effector function of NK cells. On the other hand, the research team detected that the tumor-infiltrated NK cells with Tipe2 deleted also expressed a higher level of perforin, which also depended on the presence of T-bet or Eomes, confirming the close relationship between T-bet/Eomes and the effector function of NK cells. Importantly, the team found that the inhibitory effect of deletion of Tipe2 in NK cells on tumor growth depended on the expression of T-bet, Eomes, and perforin in NK cells. If any of these three factors was lost simultaneously, the tumor resumed accelerated growth. On the other hand, the loss of Tipe2 in NK cells could further promote the antitumor effect of anti-PD-L1 therapy, suggesting a synergistic effect between the mechanisms of action ot the two (Figure 3). The study of the research team showed that TIPE2 in NK cells indirectly restricted the function of antitumor CD8+T cells by inhibiting the expression of the transcription factors T-bet, Eomes, and their downstream effector molecules, suggesting that TIPE2 is a checkpoint molecule for helper functions of NK cells.
This work was supported by programs of the National Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, Key Laboratory of Quantitative Engineering Biology, Chinese Academy of Sciences, Shenzhen Institute of Synthetic Biology, etc.
References:
[1] Bi J*, Tian Z*. NK Cell Exhaustion. Frontiers in immunology 2017, 8: 760.
[2] Bi J*, Tian Z*. NK cell dysfunction and checkpoint immunotherapy. Frontiers in immunology 2019, 10: 1999.
[3] Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R*, Tian Z*. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology2014, 59(5): 1715-1725.
[4] Bi J, Zheng X, Chen Y, Wei H, Sun R*, Tian Z*. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology 2014, 60(4): 1389-1398.
[5] Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R*, Tian Z*. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018, 19(7): 723-732.
[6] Bi J, Zheng C, Zheng X. Increased expression of adenosine A3 receptor in tumor-infiltrating natural killer cells. Cell Mol Immunol 2021, 18(2): 496-497.
[7] Bi J*, Cheng C, Zheng C, Huang C, Zheng X, Wan X, Chen YH, Tian Z, Sun H*. TIPE2 is a checkpoint of natural killer cell maturation and antitumor immunity. Science Advances, 2021, 7(38):eabi6515
[8] Bi J*, Huang C, Jin X, Zheng C, Huang Y, Zheng X, Tian Z*, Sun H*. TIPE2 deletion improves the therapeutic potential of adoptively transferred NK cells. J ImmunoTher Cancer, 2023.